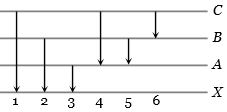
ઓરડાના તાપમાને વાયુ સ્વરૂપ હાઇડ્રોજનને પ્રતાડિત કરવા માટે $12.5\,eV$ નું ઈલેકટ્રોન પુંજ વાપરવામાં આવે છે. ઉત્સર્જિત વર્ણપટ રેખાઓની સંખ્યા $.......$ હશે.
JEE MAIN 2023, Diffcult
c
According to Bohr's postulates, an electron makes jump to higher energy orbital if it absorbs a photon of energy equal to difference between the energies of an excited state and the ground state. Assuming that collided electron takes energy equal to \(10.2\,eV\) or \(12.09\,eV\) from incoming electron beam (some part lost due to collision). The maximum excited state is \(n =3\). So, number of spectral lines is \(\frac{3(3-1)}{2}=3\)
According to Bohr's postulates, an electron makes jump to higher energy orbital if it absorbs a photon of energy equal to difference between the energies of an excited state and the ground state. Assuming that collided electron takes energy equal to \(10.2\,eV\) or \(12.09\,eV\) from incoming electron beam (some part lost due to collision). The maximum excited state is \(n =3\). So, number of spectral lines is \(\frac{3(3-1)}{2}=3\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1હાઈડ્રોજન પરમાણુમાં ધરાસ્થિતિમાં રહેલા ઇલેક્ટ્રોનની ઊર્જા $-13.6$ છે.તો ચોથી કક્ષામાં રહેલા ઇલેક્ટ્રોનની ઝડપ શોધો.View Solution
- 2અવકાશીય વિસ્તારમાં હાઇડ્રોજન અણુમાં આંતરક્રિયાને કારણે, $21\;cm$ ની તરંગલંબાઇના તરંગો ઉત્સર્જિત થાય છે, જેને હાઇડ્રોજન અણુમાં અતિસૂક્ષ્મ આંતરક્રિયા કહેવામાં આવે છે. ઉત્સર્જિત તરંગની ઊર્જા લગભગ કેટલી હશે?View Solution
- 3$H -$ પરમાણુના બોહર નમૂનામાં કોઈ પણ કક્ષાના ઈલેક્ટ્રોનની ગતિ ઊર્જા ક્વોન્ટમ આંકના સિદ્ધાંત પર .......રીતે આધારિત છે.View Solution
- 4હાઈડ્રોજન પરમાણુ તેની સ્થિતિ $\mathrm{n}=3$ માંથી $\mathrm{n}=2$ માં બદલે છે. રીકોઈલ (પાછો ધક્કો) ને કારણે ઉત્સર્જિત પ્રકાશ તરંગલંબાઈમાં ફેરફાર નું સંનિક્ટ મૂલ્ય $1 \times 10^{-n}$ મળે છે. $\mathrm{n}$ નું મૂલ્ય. . . . થશે.View Solution
[Rhc=13.6 eV, $\mathrm{hc}=1242 \mathrm{eV} \mathrm{nm}, \mathrm{h}=6.6 \times 10^{-34} \mathrm{J-s}$ અને હાઈડ્રોજન પરમાણુનું દળ $\sim 1.6 \times 10^{-27} \mathrm{~kg}$ લો.]
- 5View Solutionવર્ણપટરેખાઓની પાશ્ચન શ્રેણીમાં ટૂંકામાં ટૂંકી કઈ તરંગલંબાઈ હાજર છે?
- 6હાઈડ્રોજન પરમાણુ $975\, Å$ તરંગ લંબાઈના વિકિરણ ભૂમિ અવસ્થામાંથી ઉત્તેજીત અવસ્થામાં આવે છે. ઉત્સર્જન વર્ણપટમાં કેટલી રેખાઓ શક્ય છે?View Solution
- 7હાઈડ્રોજન પરમાણુની પ્રથમ કક્ષાની ત્રિજ્યા $ 0.5 \,Å $ અને ઈલેક્ટ્રોનની ઝડપ $2.2 ×10^6\, m /sec$ છે. તો ઈલેક્ટ્રોન ની ગતિના લીધે પ્રોટોન પાસે ઉદ્દભવતું ચુંબકીય પ્રેરણ......$Tesla$ શોધો.View Solution
- 8જ્યારે એક ક્ષ કિરણ ટ્યૂબ $60\, kV$ પર કાર્ય કરે તો ટ્યૂબ વિદ્યુત પ્રવાહનું અવલોકન $50\, mA$ છે. ધારો કે ઈલેક્ટ્રોનની કુલ ઊર્જા ઉષ્મામાં રૂપાંતરિત થાય છે. કેલોરી/સેકન્ડમાં એનોડ આગળ ઉત્પન્ન થતી ઉષ્માનું દળ ......છે.View Solution
- 9View Solutionહાઇડ્રોજન સ્પેકટ્રમની કઇ શ્રેણી દ્રશ્ય વિભાગમાં છે.
- 10નીચે આપેલ આકૃતિમાં પરમાણુ માટે ઉર્જાસ્તર અને તેના ઉત્સર્જનની છ વર્ણપટ્ટ રેખા દર્શાવેલ છે ($5$ નંબરની રેખા $ B$ સ્તરથી $A$ સ્તર પરની સંક્રાંતિ દર્શાવે છે) તો તેમાંથી કઈ વર્ણપટ્ટ રેખા શોષણ વર્ણપટ્ટમાં પણ દેખાશે?View Solution