હાઈડ્રોજન પરમાણુમાં ધરાસ્થિતિમાં રહેલા ઇલેક્ટ્રોનની ઊર્જા $-13.6$ છે.તો ચોથી કક્ષામાં રહેલા ઇલેક્ટ્રોનની ઝડપ શોધો.
AIIMS 2019, Medium
b
The ground state velocity of the electron in the hydrogen atom
The ground state velocity of the electron in the hydrogen atom
is, \(v_{0}=2.18 \times 10^{6} m / s\)
The expression for the speed of the electron in fourth orbit of \(H\) atom is,
\(v_{4 th }=v_{0} \frac{z}{n}\)
\(=2.18 \times 10^{6} \times \frac{1}{4}\)
\(=0.545 \times 10^{6} m / s\)
\(=5.45 \times 10^{5} m / s\)
Thus, the speed of the electron in fourth orbit of \(H\) -atom is \(5.45 \times 10^{5} m / s\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નબળા ક્ષ કિરણો માટે એલ્યુમિનિયમનો એટેન્યુએશન અચળાંક પ્રતિ $ 1.73 \,cm$ હોય તો $ 1.156\, cm $ જાડાઈની ઍલ્યુમિનિયમ શીટ માંથી કેટલા .......$\%$ પ્રતિશત ક્ષ કિરણો પસાર થશે ?View Solution
- 2રૂધરફોર્ડના $\alpha$ -પ્રકીર્ણનના પ્રયોગમાં ........મળે છે.View Solution
- 3હાઇડ્રોજન સ્પેકટ્રમના ઊર્જા સ્તરો આપેલા છે, $A,B$ અને $C$ કઇ શ્રેણી દર્શાવે છે.View Solution
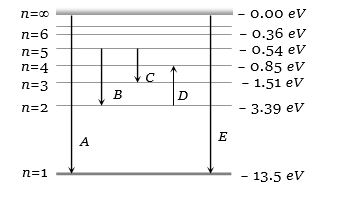
- 4પાશ્વન શ્રેણી સાથે સંકળાયેલ લાંબામાં લાંબી તરંગલંબાઈ. . . . . . હશે. $\left(\mathrm{R}_{\mathrm{H}}=1.097 \times 10^7 \mathrm{SI}\right.$ એકમ $)$View Solution
- 5હાઇડ્રોજન વર્ણપટમાં લાઇમન$(Lyman)$ અને બામર$ (Balmer)$ શ્રેણીઓની મહત્તમ તરંગલંબાઈઓનો ગુણોત્તર ........ છે.View Solution
- 6હાઇડ્રોજનની ધરા-સ્થિતિમાં રહેલા ઇલેકટ્રોનની આયનીકરણ ઊર્જા $13.6 \;eV$ છે. હાઇડ્રોજનમાં ધરા-સ્થિતિમાં રહેલ ઇલેકટ્રોનને $12.1\; eV $ ઊર્જા ધરાવતા ફોટોન વડે ઉત્તેજિત કરવામાં આવે છે. બોહરની થીયરી પ્રમાણે હાઇડ્રોજન દ્વારા ઉત્સર્જન થતી વર્ણપટ રેખાની સંખ્યા કેટલી હશે?View Solution
- 7બોહરના વાદ અનુસાર, હાઇડ્રોજન પરમાણુમાં $4^{\text {th }}$ (ચોથી) કક્ષામાં ભ્રમણ કરતાં ઇલેકટ્રોન માટે વેગમાનની ચાકમાત્રા. . . . . . . .હશે.View Solution
- 8$n=5$ થી $n=1$ કક્ષામાં સંક્રમણ દરમ્યાન ફોટોનના ઉત્સર્જનને કારણે હાઈડ્રોજન પરમાણુની પ્રતિક્ષેપ ઝડપ (recoil speed) ......... $m/s$ થશે.View Solution
- 9એક $Z$ પરમાણુક્રમાંકવાળા હાઇડ્રોજન જેવા પરમાણુમાં એક ઇલેકટ્રૉન $2n$ ક્રમાંકવાળી ઉત્તેજિત અવસ્થા છે. તેમાંથી વધુમાં વધુ $204\, eV$ ઊર્જા ધરાવતો ફોટોન ઉત્સર્જિત થઈ શકે છે. હવે આ ઇલેકટ્રૉન $2n$ કક્ષામાંથી $n$ મી કક્ષામાં સંક્રાંતિ કરે તો $40.8\, e V$ ઊર્જા ધરાવતો ફોટોન ઉત્સર્જિત થાય છે, તો $'n'$ નું મૂલ્ય .......View Solution
- 10હાઇડ્રોજન પરમાણુની ધરા-સ્થિતિમાં રહેલા ઇલેકટ્રોનની ઊર્જા $E$ છે, $L{i^{ + + }}$ માં $2^{nd}$ ઉત્તેજીત અવસ્થામાં રહેલા ઇલેકટ્રોનની ઊર્જા કેટલી થાય?View Solution
