(નજીકના પૂર્ણાંકમાં રાઉન્ડ ઑફ કરો) $[$આપેલ $: \sqrt{2}=1.41]$
Given : \(\left[ K _{ sp }\right]_{ PbI _{2}}=8 \times 10^{-9}\)
To calculate : solubility of \(PbI _{2}\) in \(0.1 \,M\) sol of \(Pb \left( NO _{3}\right)_{2}\)
\((I)\) \(Pb \left( NO _{3}\right)_{2} \rightarrow Pb _{\text {(aq) }}^{+2}+2 NO _{3}^{-}( aq )\)
\(0.1\,M\quad \quad \quad \quad \quad -\quad \quad \quad \quad \quad -\)
\(-\quad \quad \quad \quad \quad \quad \quad 0.1\,M\quad \quad \quad0.2\,M\)
\((II)\) \(PbI _{2}( s ) \rightleftharpoons Pb ^{+2}( aq )+2 I ^{-}( aq )\)
\(\quad \quad \quad \quad \quad \quad \quad \quad s\quad \quad \quad \quad \quad 2s\)
\(= s +0.1\)
\(\simeq 0.1\)
Now : \(K _{ sp }=8 \times 10^{-9}=\left[ Pb ^{+2}\right][ I^- ]^{2}\)
\(\Rightarrow 8 \times 10^{-9}=0.1 \times(2 s )^{2}\)
\(\Rightarrow 8 \times 10^{-8}=4 s ^{2} \Rightarrow s =\sqrt{2} \times 10^{-4}\)
\(\Rightarrow S =141 \times 10^{-6} \,M\)
\(\Rightarrow x =141\)
Download our appand get started for free
Similar Questions
- 1જો લેકટીક એસિડની $pKa\,5$ હોય તો, $25^{\circ}\,C$ પર $0.005\,M$ કેલ્શીયમ લેકટેટ દ્રાવણની $pH ................. 10^{-1}$ છે.View Solution
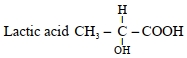
- 2$AgCl$ ની દ્રાવ્યતા ઓછામાં ઓછી કેમાં હશે?View Solution
- 3View Solutionનીચે આપેલા વિકલ્પો પૈકી નીચેના અણુઓ/આયનમાંથી કયો લુઈસ ઍસિડ તરીકે વર્તે છે?
- 4બેઝિક બફર [$e.g. NH_4OH/NH_4Cl]$ ની $pOH \,5$ છે. જો ક્ષારની સાંદ્રતા ત્રણ ગણી, જ્યારે બેઇઝ અચળ છે. તો નવા $pOH$ નું મૂલ્ય શોધો ? (આપેલ $log\, 3 = 0.48$)View Solution
- 5નિર્બળ વિદ્યુત વિભાજ્ય માટે વિયોજન અંશ $= .......$View Solution
- 6$HSO_3^-$નો સંયુગ્મ બેઈઝ ...... છે.View Solution
- 7અલ્પ દ્રાવ્ય ક્ષાર માટે ${A_p}{B_q}$ તેના દ્રાવ્યતા ગુણાકાર $({L_S})$નો તેની દ્રાવ્યતા $(S)$ સાથે સંબંધ શું છે?View Solution
- 8$90\,^oC$, એ $0.1\,M$ $NaCl$ જલીય દ્રાવણની $pH =$ .......View Solution
- 9નિર્બળ એસિડ અને નિર્બળ બેઈઝમાંથી બનતા ક્ષાર માટેનું $pK_h$ મુલ્યનું સાચું સૂત્ર .....View Solution
- 10$298\,K$ તાપમાને $NaCl$ ના દ્રાવણની $p^H = 7$ છે. જો દ્રાવણને $320\,K$ તાપમાન સુધી ગરમ કરવામાં આવે, તો નીચેના પૈકી ક્યુ વિધાન સાચું છે ?View Solution
