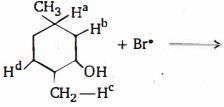
પેરોક્સાઇડની હાજરીમાં, હાઇડ્રોજન ક્લોરાઇડ અને હાઇડ્રોજન આયોડાઇડ આલ્કીન્સમાં પ્રતિ-માર્કોવનિકોવ યોગશીલ આપતા નથી કારણ કે
IIT 2001, Medium
c
$HI$ and $HCl$ do not do this for energetic reasons.
$HI$ and $HCl$ do not do this for energetic reasons.
The addition of $Cl$ and $I$ radicals to alkanes in an anti-Markovnikov fashion is an endothermic reaction and therefore counterproductive.
Without the presence of peroxide, all hydrogen halides will add according to the Markovnikov rule.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1ઇથાઇલ આલ્કોહોલને લાલ ફોસ્ફરસ અને $HI$ સાથે ગરમ કરતા શું મળે છે ?View Solution
- 2નીચેનામાંથી કઈ નીપજએ $ o $-xylene ના ઑઝોનોલિસિસમાં મેળવી શકાતી નથી ?View Solution
- 3$C{H_3} - \mathop {CH}\limits^{\mathop |\limits^{C{H_3}} } \,\, - \,\,C{H_2} - C{H_3}\,\,\xrightarrow{{C{l_2}/hv}}\,\,N$( સમઘટકોની સંખ્યા) $ \xrightarrow{{Fractional\,\,\,{\text{distillation}}}}\,\,(F)\,,\,\,(N)$ અને $\,(F)\,$ એ .............View Solution
- 4(વલય નું વિસ્થાપન નથી ) નીપજ $(A)$ કઈ હશે ?View Solution
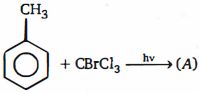
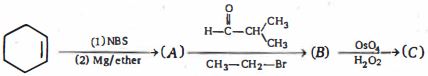
- 5નીપજ $(C)$ શું હશે ?View Solution
- 6$3$-ફિનાઈલ પ્રોપીન પર $HBr$ સાથે પ્રક્રિયા કરતા (મુખ્ય નીપજ) શું મળે છે ?View Solution
- 7$CH _{3}- C \equiv CH \stackrel{2 HBr }{\longrightarrow} \stackrel{ H _{2} O }{\longrightarrow}$ નીપજ, નીપજ કઈ છે:View Solution
- 8View Solutionમિથેન અને ક્લોરિનની પ્રકિયા મિશ્રણમાં ઓક્સિજનનો ઉમેરો ............... (photochemical chlorination)
- 9નીચેની પ્રક્રિયાઓના ક્રમમાં એક જ સમતલમાં $'C$'માં અણુઓની મહત્તમ સંખ્યા હાજર છેView Solution
$A \xrightarrow[ { Cu\; tube }]{\text { Redhot }}\mathrm{B} \xrightarrow[ Anhydrous AlCl_3]{\mathrm{CH}_{3} \mathrm{Cl}(1 \mathrm{eq}} \mathrm{C}$
($A$ એ સૌથી ઓછું પરમાણ્વીય વજન ધરાવતું આલ્કાઇન છે)
- 10$Br^\bullet $હાઇડ્રોજનમાંથી કયાને સરળતાથી સહન કરશે?View Solution