$CH _4+ Cl _2 \rightarrow CH _3 Cl + HCl$
The above reaction is an example of Chlorination. Chlorination is the substitution of hydrogen atoms by chlorine atom.
The conditions used in the above process is $U.V.$ light or diffused sunlight or $400^{\circ} C$.
This reaction is an example of substitution reaction.
Download our appand get started for free
Similar Questions
- 1View Solutionનીચેની પ્રક્રિયાઓ ધ્યાનમાં લો, અને સાચી પ્રક્રિયાઓ કઈ છે?
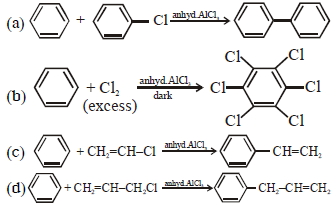
- 2નીચેની આપેલી પ્રક્રિયાઓના ક્રમમાં મુખ્ય નીપજ $[B]$ શું હશે ?View Solution
$\begin{array}{*{20}{c}}
{C{H_3} - C = CH - C{H_2}C{H_3}} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{CH{{(C{H_3})}_2}\,\,\,\,\,\,\,\,\,\,}
\end{array}\xrightarrow[{(ii)\,{H_2}{O_2},O{H^ - }}]{{(i)\,{B_2}{H_6}}}[A]$$\xrightarrow[\Delta ]{{dil.\,{H_2}S{O_4}}}[B]$ - 3View Solutionનીચેનામાંથી કોનો ઓક્ટેન આંક સૌથી ઓછો છે ?
- 4નિર્જળ ઝિંક ક્લોરાઇડની હાજરીમાં $2-$મિથાઇલ પ્રોપીન સાથે એસિટાઇલ ક્લોરાઇડથી ગરમ કરતાં કાર્બનિક સંયોજન નિર્માણ પામે છે,તેનું સૂત્ર શું હશે?View Solution
- 5View Solutionનીચે આપેલી પ્રકિયા ની નીપજ શું હશે ?
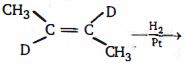
- 6નીચેની પ્રકિયા ની મુખ્ય નીપજ કઈ છે ?View Solution
$\begin{array}{*{20}{c}}
{\begin{array}{*{20}{c}}
{\,\,\,\,\,\,\,C{H_3}} \\
|
\end{array}} \\
{C{H_3} - C - C{H_3}} \\
| \\
H
\end{array}{\mkern 1mu} $ $\mathop {\xrightarrow{{C{H_3}OBr}}}\limits_{C{H_3}OH} $ - 7આપેલ ઉપરની પ્રક્રિયાને ધ્યાનમાં લો. નીપજ $(P)$ ના પ્રતિઅણુ હાજર ઓકિસનન પરમાણુઓની કુલ સંખ્યા________છે.View Solution
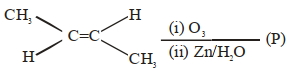
- 8$(B)$ અને $(C)$ વચ્ચે ની પ્રકિયા શું થશે ?View Solution
$C{H_3} - C \equiv C - H\xrightarrow{{NaN{H_2}}}\xrightarrow{{C{H_3} - I}}(A)\xrightarrow{{Li/liq\,N{H_2}}}(B)$
$C{H_3} - C \equiv C - H\xrightarrow{{NaN{H_2}}}\xrightarrow{{C{H_3} - I}}(A)\xrightarrow[{Pd.CaC{O_3}}]{{{H_2}}}(C)$
- 9View Solutionએરોમેટીક અણુઓ...... ધરાવતા નથી ?
- 10$C{{H}_{3}}CH\,=\,\,C{{H}_{2}}\,\underset{700\,K}{\mathop{\xrightarrow{C{{l}_{2}}}}}\,\,A\,\underset{420\,K,\,12\,atm}{\mathop{\xrightarrow{N{{a}_{2}}C{{O}_{3}}}}}\,\,\,B\,\,\underset{(ii)\,NaOH}{\mathop{\xrightarrow{(i)\,HOCl}}}\,\,\,C$View Solution
પ્રક્રિયામાં શ્રેણી ને ધ્યાનમાં રાખતા પદાર્થ $C$ શું હશે?3
