આપેલ : $\Delta H _{ f }{ }^\theta\left( Al _2 O _3\right)=-1700\,kJ\,mol ^{-1}$
$\Delta H _{ f }{ }^\theta\left( Fe _2 O _3\right)=-840\,kJ\,mol ^{-1}$
$Fe , Al$ અને $O$ નું મોલર દળ અનુક્રમે $56,27$ અને $16\,g\,mol ^{-1}$.
$Fe _2 O _3+2 Al \rightarrow Al _2 O _3+2 Fe$
Molar mass $160\,g \quad 27\,g$
$\left(\Delta H _{ f }^0\right)_{\text {reaction }}=\left[\left(\Delta H _{ f }^0\right)_{ Al _2 O _3}+2\left(\Delta H _{ f }^0\right)_{ Fe }\right]- \left[\left(\Delta H _{ f }^0\right)_{ Fe _2 O _3}+2\left(\Delta H _{ f }^0\right)_{ Al }\right]$
$=[-1700+0]-[-840+0]$
$=-860\,kJ / mol$
$\text { Total mass of mixture }= Fe _2 O _3+ Al (1: 2 \text { molar }$
$\text { ratio })$
$=160+2 \times 27$
$=214\,g / mol$
$\text { Heat evolved per gram }=\frac{860}{214}=4\,kJ / g$
Download our appand get started for free
Similar Questions
- 1નીચેના પૈકી ક્યા સમીકરણ માટે $\Delta H_{{\rm{react}}}^o$ નું મૂલ્ય નીપજ માટેના $\Delta H_f^o$ જેટલું હશે ?View Solution
- 2$298\,K$ એ $N_2$$_{(g)}$ + $3H_2$$_{(g)}$ $\rightarrow$ $2NH_3$$_{(g)}$ પ્રક્રિયા માટે એન્થાલ્પી પરિવર્તન $\Delta H - 92.38\,KJ$ તો $298\,K$ એ $\Delta U$ કેટલા .....$kJ$ થાય ?View Solution
- 3નીચેના પ્રકમો પરથી પ્રકિયા $B + D \rightarrow E + 2C$ માટે $\Delta H$ નું મૂલ્ય કેટલા ........... $\mathrm{kJ/mol}$ હશે તે જણાવો.View Solution
$\Delta H \,(kJ/mol)$ $\frac 12 A \rightarrow B$ $+150$ $3B \rightarrow 2C + D$ $-125$ $E + A \rightarrow 2D$ $+350$ - 4$PH_3$$_{(g)}$ નું એટોમીકરણ ઉષ્મા $228 \,K\,cal\, mol^{-1}$ અને $P_2H_4$ ની $ 355 \,K\,cal \,mol^{-1}$ છે. $P - P$ બંધની બંધ ઉર્જા .......($K\,cal$ માં)View Solution
- 5View Solutionજ્યારે વાયુ સમોષ્મી અને પ્રતિવર્તીં રીતે સંકોચાય ત્યારે અંતિમ તાપમાન.......
- 6નિર્જળ $\mathrm{CuSO}_4$ અને $\mathrm{CuSO}_4 \cdot 5 \mathrm{H}_2 \mathrm{O}$ ના દ્રાવણ ની ઉષ્મા અનુક્રમે $-70 \mathrm{~kJ} \mathrm{~mol}^{-1}$ અને $+12 \mathrm{~kJ} \mathrm{~mol}^{-1}$ છે. $\mathrm{CuSO}_4$ થી $\mathrm{CuSO}_4 \cdot 5 \mathrm{H}_2 \mathrm{O}$ ની જલીયકરણ ની ઉષ્મા $-x \mathrm{~kJ}$ છે. તો $x$ નું મૂલ્ય. . . . . . . .છે. (નજીકનો પૂર્ણાક)View Solution
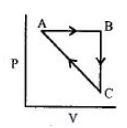
- 7એક આદર્શ વાયુ નીચે દર્શાવેલ આકૃતિ મુજબ ચક્રીય પ્રક્રમ અનુભવે છે.View Solution
$\Delta {U_{BC}} = - 5\,kJ\,mo{l^{ - 1}},{q_{AB}} = 2\,kJ\,mo{l^{ - 1}}$
$\Delta {W_{AB}} = - 5\,kJ\,mo{l^{ - 1}},{W_{CA}} = 3\,kJ\,mo{l^{ - 1}}$
$CA$ પ્રક્રમ દરમિયાન પ્રણાલી દ્વારા શોષાતી ઉષ્મા ......$kJ\,mo{l^{ - 1}}$
- 8પાણીની બાષ્પાયન એન્થાલ્પી $186.5 \,KJ\, mol^{-1}$ છે. તો તેનાં બાષ્પાયનની એન્ટ્રોપી કેટલા......$KJ\,K^{-1} \,mol^{-1}$ થશે ?View Solution
- 9કાર્બનના દહનથી બે ઓક્સાઈડ અનુક્રમે $CO$ અને $CO_2$ બને છે. તેઓની સર્જન એન્થાલ્પી અનુક્રમે $26$ કિલોકેલરી અને $94.3$ કિલોકેલરી છે, તો કાર્બનની દહન એન્થાલ્પી ...... કિલોકેલરી થાય.View Solution
- 10View Solutionસમોષ્મી પ્રક્રિયા માટે નીચેનામાંથી કયો સંબંધ સાચો છે ?
