$\Delta {U_{BC}} = - 5\,kJ\,mo{l^{ - 1}},{q_{AB}} = 2\,kJ\,mo{l^{ - 1}}$
$\Delta {W_{AB}} = - 5\,kJ\,mo{l^{ - 1}},{W_{CA}} = 3\,kJ\,mo{l^{ - 1}}$
$CA$ પ્રક્રમ દરમિયાન પ્રણાલી દ્વારા શોષાતી ઉષ્મા ......$kJ\,mo{l^{ - 1}}$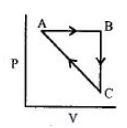
$\Delta {U_{AB}} = {q_{AB}} + {W_{AB}} = 2 + ( - 5) = - 3\,kJ/mol$
$\Delta {U_{BC}} = - 5\,kJ/mol$
For cyclic procrss, $\Delta U=0$
$\Delta {U_{AB}} + \Delta {U_{BC}} + \Delta {U_{CA}} = 0$
$\Delta {U_{CA}} = - \Delta {U_{AB}} - \Delta {U_{BC}}$
$\Delta {U_{CA}} = - ( - 3) - ( - 5) = 8\,kJ/mol$
$\Delta {U_{CA}} = {q_{CA}} + {W_{CA}}$
$8 = {q_{CA}} + 3$
${q_{CA}} = + 5\,kJ/mol$
Heat absorbed has positive sign.
Download our appand get started for free
Similar Questions
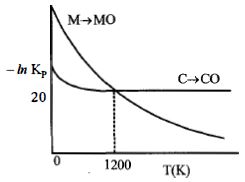
- 1નીચેનો આલેખ એ પ્રક્રિયાઓ $M(s) + \frac{1}{2}{O_2}(s)\, \to \,MO(s)\,$ અને $C(s) + \frac{1}{2}{O_2}(g)\, \to \,CO(s)\,$ માટે $- In\,K_p$ વિરુદ્ધ તાપમાનનો ફેરફાર દર્શાવે છે. તો સાયુ વિધાને ઓળખો.View Solution
- 2જયારે $60\,W$ ઈલેકિટ્રક હીટર ને વાયુમાં $100\,s$ માટે સમોષ્મી દિવાલો સાથે સમોષ્મી સાથે ના અચળ કદ ના પાત્રમાં $100\,s$માટે ડુબાડવામાં આવે છે.ત્યારે વાયુ નું તાપમાન $5^{\circ}\,C$ વધે છે.આપેલ વાયુ ની ઉષ્માક્ષમતા $........\,J\,k ^{-1}$ છે.(નજીકનો પૂર્ણાક)View Solution
- 3નીચેની માહિતી પરથી રહોમ્બિક સલ્ફર $\left( {{S_R}} \right)$ માંથી મોનોક્લિનિક સલ્ફર $\left( {{S_M}} \right)$ ની સંક્રાંતિ ઉષ્મા ....$kJ$ થશેView Solution
${S_R} + {O_{2\left( g \right)}} \to S{O_{2\left( g \right)}};\,\Delta H = - 296.90\,kJ$
${S_M} + {O_{2\left( g \right)}} \to S{O_{2\left( g \right)}};\,\Delta H = - 299.40\,kJ$
- 4પ્રાણાલીની આંતરિક ઉર્જા $U_1$ છે, તો બહારથી $450\, J$ ઉષ્મા લે છે અને $600\, J$ કાર્ય પુરૂ કરે છે તો પ્રાણાલીની અંતિમ ઉર્જા .......View Solution
- 5પદાર્થની $\Delta H_{fusion}$ $'x'$ છે અને $\Delta H_{vap}$ $'y'$ છે, પછી $\Delta H_{sublimation}$ હશે,.....View Solution
- 6$298\, K$ તાપમાને અને $1$ વાતાવરણ દબાણે $CaCO_3 { }_{(s)}$ $\rightarrow CaO { }_{(s)} + $ $CO_2$$_{(g)}$ પ્રક્રિયામાં $\Delta H^o$ અને $\Delta S^o$ નાં મૂલ્યો અનુક્રમે $+179.1$ કિલો જૂલ મોલ$^{-1}$ અને $+160.2$ જૂલ $K ^{-1}$ છે. ધારો કે તાપમાન બદલાતાં $\Delta H^o$ અને $\Delta S^o$ નાં મૂલ્યો બદલાતાં નથી, તો આ પ્રક્રમ ......$K$ તાપમાનથી ઊંચા તાપમાન આપમેળે થશે ?View Solution
- 7View Solutionપદાર્થની આંતરિક ઉર્જા શું હશે ?
- 8પ્રતિમોલ ઈથેનોલની બાષ્પાયન એન્થાલ્પી કેટલા ............ $\mathrm{kJ/mol}$ થશે ? $(b.p. = 79.5\,^oC$ અને $\Delta S$ $= 109.8 $ $JK^{-1}\, mol^{-1}$) છે.View Solution
- 9ઓટોમોબાઈલમાં થતી નીચેની પ્રક્રિયા માટે $\Delta H, \Delta S, \Delta G$ નાં મૂલ્યો અનુક્રમે ...... હોય છે.View Solution
$2C_8H_{18}$ $_{(g)}$ + $25O_2$ $_{(g)}$ $\rightarrow$ $16CO_2$$_{(g)}$ + $18H_2O$ $_{(g)}$
- 10નીચેનામાંથી ક્યા ઉષ્માગતિકીય પ્રકમ માટે $\Delta U = 0$ થશે ?View Solution
