સમોષ્મી પ્ર્ક્રિયા દરમ્યાન, વાયુનું દબાણ તેના નિરર્પેક્ષ તાપમાનના ઘનના સમપ્રમાણમાં માલૂમ પડે છે, તો વાયુ માટે $\frac{C_P}{C_V}$ ગુણોત્તર. . . . . . . .હશે.
JEE MAIN 2024, Diffcult
c
\(\mathrm{P} \propto \mathrm{T}^3\)
\(\mathrm{P} \propto \mathrm{T}^3\)
\(\mathrm{PT}^{-3}=\text { constant }\)
\(\because \frac{\mathrm{PV}}{\mathrm{T}}=\mathrm{nR}=\text { constant from ideal gas equation }\)
\((\mathrm{P})(\mathrm{PV})^{-3}=\text { constant }\)
\(\mathrm{P}^{-2} \mathrm{~V}^{-3}=\text { cosntant } \quad . .(1)\)
\(\because \text { Process equation for adiabatic process is }\)
\(\mathrm{PV}^y=\text { constant } \quad . .(2)\)
\(\text { Comparing equation (1) and (2) }\)
\(\frac{\mathrm{C}_{\mathrm{p}}}{\mathrm{C}_{\mathrm{V}}}=\mathrm{y}=\frac{3}{2}\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1એક દ્વિપરમાણ્વિક વાયુ $P V^{1.3}=$ અચળ વડે રજૂ કરેલ પ્રક્રિયામાંથી પસાર થાય છે. સાચુ નિવેદન પસંદ કરો.View Solution
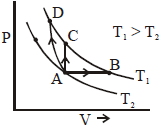
- 2એક પરમાણ્વિક આદર્શ વાયુ માટેની ત્રણ અલગ-અલગ પ્રક્રિયા માટે $P$ વિરુધ્ધ $V$ નો ગ્રાફ આપેલ છે. તેમના પથને $A \rightarrow B, A \rightarrow C$ અને $A \rightarrow D .$ વડે દર્શાવેલ છે. આ પ્રક્રિયા દરમિયાન થતો આંતરિક ઉર્જાનો ફેરફાર $E _{ AB }, E _{ AC }$ અને $E _{ AD }$ અને થતાં કાર્યને $W _{ AB }$ $W _{ AC }$ અને $W _{ AD }$ વડે દર્શાવેલ છે. તો આપેલ પરિણામો વચ્ચે સાચો સંબંધ શું થશે?View Solution
- 3View Solutionસમતાપી પ્રક્રિયા માટે કયું વિધાન ખોટું છે.
- 4કાર્નોટ એન્જિનની કાર્યક્ષમતા $40\%$ હોય,જયારે ઠારણ વ્યવસ્થાનું તાપમાન $500$ $K$ હોય છે. કાર્યક્ષમતા $60\%$ કરવા માટે ઉષ્મા પ્રાપ્તિસ્થાનનું તાપમાન અચળ રાખીને ઠારણ વ્યવસ્થાનું તાપમાન ....... $K$ કરવું પડે?View Solution
- 5સમોષ્મી પ્રક્રિયામાં $1 \,\,mol$ આદર્શ વાયુનું પ્રારંભિક અને અંતિમ તાપમાન અનુક્રમે $T_1$ અને $T_2$ છે, તો વાયુની આંતરિક ઊર્જામાં થતો ફેરફાર .......View Solution
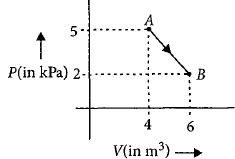
- 6એક મોલ દ્વિ પરમાણ્વિક આદર્શ વાયુ $A$ થી $B $ પર આકૃતિમાં બતાવ્યા મુજબ $AB $ માર્ગે જાય છે. આ ગતિમાં વાયુની આંતરિક ઊર્જાનો ફેરફાર ...............$kJ$ હશે.View Solution
- 7$PV$ ડાયાગ્રામ દ્વારા રજૂ કરેલી નીચેની કઈ થરમોડાયનેમિક પ્રક્રિયા દરમિયાન તંત્ર દ્વારા શોષેલી ઉષ્મા ઊર્જા એ $PV$ આલેખ દ્વારા આવરીત ક્ષેત્રફળ જેટલી હોય છે.View Solution
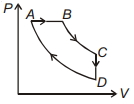
- 8આકૃતિંમાં દર્શાવ્યા મુજબ થરમોડાયનેમિક ચક્રના $P-V$ ડાયાગ્રામ માટે, પ્રક્રિયા $B C$ અને $D A$ સમતાપી છે. તેને અનુરૂપ નીચેનામાંથી ક્યું આલેખ સાચું છે ?View Solution
- 9View Solutionવિધાન : ઉષ્મીય રીતે અલગ કરેલ તંત્રમાં એન્ટ્રોપી વધે
કારણ : ઉષ્મીય રીતે અલગ કરેલ તંત્રમાં સમોષ્મિ પ્રક્રિયા થાય
- 10આદર્શ વાયુને સમોષ્મી રીતે સંકોચન કરતાં તેની ઘનતા પહેલા કરતાં $32$ ગણી થાય છે.જો અંતિમ દબાણ $128\,atm$ હોય તો વાયુ માટે $\gamma $ નું મૂલ્ય કેટલું હશે?View Solution
