$STP$ એ સાર્વત્રિક વાયુ અચળાંક ...... $J mol^{-1} K^{-1}$ શોધો.
Easy
b
સાર્વત્રિક વાયુ અચળાંક \(R\,\, = \,\,\,\frac{{PV}}{T}\)
સાર્વત્રિક વાયુ અચળાંક \(R\,\, = \,\,\,\frac{{PV}}{T}\)
\(S.T.P.\) એે બધા જ વાયુના એક મોલનું \(V = 22.4\, litre = 22.4 \times 10^{-3} m^3\)
\(P = 760 \,mm\, of\, Hg = 760 \times 10^{-3} \times 13.6 \times 10^3 \times 9.80 N m^{-2}\,\,\, T = 273 K\)
\(\therefore \,\,R\,\, = \,\,\frac{{760\,\, \times \,\,{{10}^{ - 3}} \times \,\,13.6\,\, \times \,\,{{10}^3} \times \,\,9.80\,\, \times \,\,22.4\,\,\, \times \,\,\,{{10}^{ - 3}}}}{{273}}\,\,\, = \,\,\,8.31\,\,J\,\,mo{l^{ - 1}}\,\,{K^{ - 1}}\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચેનામાંથી કેટલા $K$ તાપમાને હાઈડ્રોજન પરમાણુની $r.m.s$ વેગ એ પૃથ્વીના નિષ્ક્રમણ વેગ જેટલો થાય ?View Solution
- 2એક પાત્રને બે ચેમ્બરમાં વિભાજીત કરવામાં આવે છે જ્યાં પ્રથમ ચેમ્બરનું કદ $4.5$ લીટર અને બીજા ચેમ્બરનું કદ $5.5$ લીટર છે. પ્રથમ ચેમ્બર $2.0\, atm$ દબાણે $3.0$ મોલ વાયુ ધરાવે છે તેમજ $3.0\, atm$ દબાણે બીજે ચેમ્બર $4.0$ મોલ વાયું ધરાવે છે. જ્યારે બે ચેમ્બર વચ્ચે થી વિભાજન (પાર્ટીશન) ને દૂર કરવામાં આવે ત્યારે મિશ્રણ સંતુલન પ્રાપ્ત કરે છે. આ મિશ્રણમાં ઉદભવતા દબાણનું મૂલ્ય $x \times 10^{-1} \,atm$ છે. 1 નું મૂલ્ય ........ છે.View Solution
- 3કોઈ વાયુનો $27°C$ ઓરડાના તાપમાને $rms$ વેગ $ 412 m/s$ મળે છે. આ વાયુનું નામ......છે.View Solution
- 4વાયુનું દબાણ $6 \times 10^4 N/m^{2}$ છે. એકમ કદ દીઠ પરમાણુઓની ગતિ ઊર્જા શું થશે?View Solution
- 5$ 27^\circ C $ તાપમાને વાયુનું કદ $V$ છે,તો દબાણ અચળ રાખીને વાયુનું તાપમાન $ 327^\circ C $ કરવામાં આવે,તો વાયુનું નવું કદ કેટલું થાય?View Solution
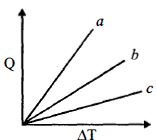
- 6એક પરમાણ્વિક $(M)$, દ્વિ પરમાણ્વિક $(D)$ અને બહુ પરમાણ્વિક $(P)$ વાયુઓની સમદાબી પ્રક્રિયા માટે આપેલી ઉષ્મા$(Q)$ અને તાપમાનના થતાં ફેરફાર$\left( {\Delta T} \right)$ વચ્ચેનો ગ્રાફ આપેલ છે.શરૂઆતમાં બધા જ વાયુ સમાન છે જો કંપનગતિ માટે મુક્તતાના અંશોને અવગણવામાં આવે તો $a, b$ અને $c$ ગ્રાફની રેખા કોને અનુરૂપ હશે?View Solution
- 7એક આદર્શ વાયુ માટે અચળ દબાણે મોલર વિશિષ્ટ ઉષ્મા $(7/2) R$ છે. અચળ દબાણે વિશિષ્ટ ઉષ્મા અને અચળ કદે વિશિષ્ટ ઉષ્માનો ગુણોત્તર કેટલો થાય?View Solution
- 8$23°C$ ઓરડાના તાપમાને $37°C$ તાપમાનવાળો માણસ $1500\, ml$ વાયુને શ્વાસમાં ગ્રહણ કરે છે. જો દબાણ અને દળ અચળ હોય તો માણસના ફેફસામાં રહેલા વાયુનું કદ .... $ml$ હશે.View Solution
- 9એક પરમાણ્વિક વાયુના એક મોલની ગતિઊર્જા $0\,°C$ તાપમાને કેટલી હોય? $(R = 8.31 J/mole-K)$View Solution
- 10$0.056\, kg$ દળ ધરાવતા નાઈટ્રોજનને પાત્રમાં $127^{\circ} C$ તાપમાને બંધ રાખવામાં આવેલ છે. તેના પરમાણુઓની ઝડપ બમણી કરવા માટે જરૂરી ઉષ્મા .....$k cal$ હશે.View Solution
( $R =2 cal mole { }^{-1} K ^{-1}$ લો.)
