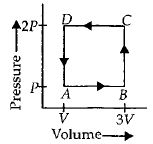
થર્મોડાયનેમિક તંત્ર આકૃતિમાં બતાવ્યા પ્રમાણે $ABCD$ ચક્રમાંથી પસાર થાય છે. આ ચક્રમાં વાયુ વડે છોડવામાં આવતી ઉષ્મા કેટલી હશે?

AIPMT 2012, Easy
a
In a cyclic process,
In a cyclic process,
\(\Delta U = 0\)
In a cyclic process work done is equal to the area under the cycle and is positive if the cycle is clockwise and negative if anticlockwiase.
\(\therefore \,\,\,\Delta W = - Area\,of\,rectangle\,ABCD = - P\left( {2V} \right)\)
\( = - 2PV\)
According to first law of thermodynamics
\(\Delta Q = \Delta u + \Delta W\,or\,\Delta Q = \Delta W\,\,\left( {As\,\Delta u = 0} \right)\)
\(i.e.,\) heat supplied to the system is equal to the work done
So heat absorbed,\(\Delta Q = \Delta W = - 2PV\)
\(\therefore \) Heat rejected by the gas \( = 2PV\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1કાર્નોટ એન્જિનની કાર્યક્ષમતા $100\%$ હોતી નથી,કારણ કેView Solution
- 2View Solutionએક પ્રતિવર્તી એન્જિન અને એક અપ્રતિવર્તી એન્જિન સમાન તાપમાનો વચ્ચે કાર્ય કરે છે, તો તેમની કાર્યક્ષમતા ... છે.
- 3એક આદર્શ ઉષ્મીય યંત્ર માટે, સ્ત્રોતનું તાપમાન $127\,^{\circ} C$ છે. $60\, \%$ જેટલી કાર્યક્ષમતા મેળવવા માટે, ઠારણનું તાપમાન $........\,{ }^{\circ} C$ હોવું જોઈએ. (નજીકત્તમ પૂર્ણાકમાં લખો)View Solution
- 4તંત્ર અવસ્થા $i$ માંથી અવસ્થા $f$ માં $iaf$ માર્ગ માટે $ Q = 50\,J $ અને $ W = 20J. $ છે. માર્ગ $ibf$ માટે $ Q = 35J. $ છે. માર્ગ $fi$ માટે $ W = - 13J $ હોય,તો $Q =$........ $J$View Solution
- 5અવાહક પાત્રમાં $4 \,mol$ આદર્શ દ્વિ પરમાણ્વિક વાયુ $T$ તાપમાને ભરેલ છે.વાયુને $Q$ ઉષ્મા આપતાં $2\, mol$ વાયુનું એક પરમાણ્વિક વાયુમાં રૂપાંતર થાય છે.જો તાપમાન અચળ રહેતું હોય,તો ઉષ્મા $Q$ કેટલી હશે?View Solution
- 6કાર્નોટ એન્જિન $400\, K$ અને $800\, K$ વચ્ચે કાર્ય કરે છે. ચક્ર દીઠ કાર્ય $1200\, J$ હોય તો ચક્ર દીઠ એન્જિનને અપાતી ઉષ્મા .......... $J$ હશે.View Solution
- 7અચળ દબાણ $P$ એ વાયુનું કદ $ {V_1} $ થી વધારીને $ {V_2} $ કરવામાં આવે છે.તો વાયુ પર થતું કાર્ય?View Solution
- 8કાર્નોટ એન્જિનની $800 K$ થી $500 K$ અને $x\, K$ થી $600\, K$ વચ્ચે કાર્યક્ષમતા સમાન છે,તો $x=$ ........ $K$View Solution
- 9View Solutionઆપેલ ગ્રાફમાં ચાર પ્રક્રિયા આપેલ છે સમકદ,સમદાબી,સમતાપી અને સમોષ્મિ પ્રક્રિયાનો સાચો ક્રમ નીચેનામાથી કયો થશે?
- 10સમાન ક્ષમતા ધરાવતા બે નળાકારો $A$ અને $B$ ને એક બીજા સાથે એક સ્ટોપ કોક થી જોડેલ છે $A$ એક પ્રમાણભૂત તાપમાન અને દબાણે એક આદર્શ વાયુ ધરાવે છે $B$ સંપૂર્ણ ખાલી છે આ આખી પ્રણાલી ઉષ્મીય અવાહક છે આ સ્ટોપ કોકને અચાનક ખોલવામાં આવે છે આ પ્રક્રિયા ........... છે.View Solution
