Question Bank
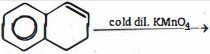
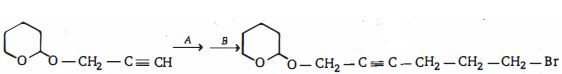
Explore our large set of questions to practice for your standard seamlessly- 1View Solutionઆ પ્રકિયા ની નીપજ શું હશે ?
- 2આ પ્રકિયા માં $Me -C \equiv C - Et \xrightarrow{{Na/liq.N{H_3}}}P\xrightarrow[{CC{l_4}}]{{B{r_2}}}(Q)$ ; $Q$ શું હશે ?View Solution
- 3આ પ્રકિયા માં નીપજ $(B)$ શું છે ?View Solution
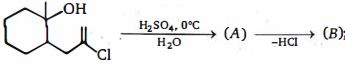
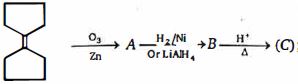
- 4આ પ્રકિયા માં નીપજ $(C)$ શું હશે ?View Solution
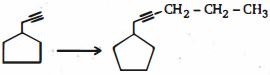
- 5View Solutionઆમાંથી કયું એરિન માટે સાચું છે?
- 6View Solutionઆ રૂપાંતરણ કોના દ્વારા મેળવવામાં આવે છે ?
- 7View Solutionઆલ્કલાઈન હેલાઈડ એ ડાયઆલ્કાઈલ કોપર સાથે પ્રક્રિયા કરીને કયો પ્રક્રિયક આપે છે ?
- 8આલ્કાઇન $KMnO_4$ દ્રાવણનો રંગ દૂર કરતું નથી તથા ઍમોનિયમ સિલ્વર નાઇટ્રેટ સાથે અવક્ષેપ આપતું નથી આ હાઇડ્રોકાર્બન ....... છે.View Solution
- 9View Solutionઆલ્કાઇનમાંથી આલ્કીનની બનાવટ માટે...... યોગ્ય ગણાય.
- 10View Solutionઆલ્કાઇન સંયોજનો....... પ્રક્રિયા દર્શાવી શકે.
- 11View Solutionઆલ્કિન ન આપતી હોય તેવી પ્રક્રિયા જણાવો.
- 12આલ્કિનમાંથી $Al_2O_3$ નો ઉપયોગ કરી આલ્કોહોલ બનાવવામાં અસરકારક કયું પરિબળ છે ?View Solution
- 13આલ્કીન $(A)$ એ $H_2$ ના $3\, mole$ સાથે જોડાઈને પ્લેટિનમ ઉદીપક ની હાજરી માં $1$આઇસોપ્રોપાઇલ - $4$ -મિથાઇલ સાયકલોહેકઝેન બનાવે છે આલ્કીન એ આયોનાઈઝ અને ઘટે છે તો નીચેનામાંથી કઈ નીપજ મળશે ?View Solution
$\begin{matrix}
O \\
|| \\
H-C-H, \\
\end{matrix}\begin{matrix}
O\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,O\,\,\,\,\,\,\,O\,\,\,\, \\
||\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,||\,\,\,\,\,\,\,\,\,||\,\,\,\,\, \\
H-C-C{{H}_{2}}-C-C-C{{H}_{3}}, \\
\end{matrix}\begin{matrix}
\,\,\,\,\,O\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,O \\
\,\,\,\,\,\,||\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|| \\
C{{H}_{3}}-C-C{{H}_{2}}-C-H \\
\end{matrix}$આલ્કેન$(A)$ શું હશે ?
- 14View Solutionઆલ્કીનના હાઈડ્રેશનમાં એસિડ ઉદ્દીપક તરીકે હોય છે જેમાં ઈથીન સિવાય......નું નિર્માણ થાય છે ?
- 15View Solutionઆલ્કીનની બનાવટ માટે કાર્બોક્સિલિક એસિડના સોડિયમ કે પોટેશિયમ ક્ષારના જલીય સંતૃપ્ત દ્રાવણનુ ........ કરવામાં આવે છે.
- 16View Solutionઆલ્કીન (પેરોક્સાઇડ્સની ગેરહાજરીમાં) સાથે હાઇડ્રોજન બ્રોમાઇડની પ્રતિક્રિયામાં, પ્રતિક્રિયાનું પ્રથમ તબબ્કો એ ............ છે
- 17View Solutionઆલ્કેન (સાયકલોઆલ્કેન નથી ) એ કાર્બનિક અણુઓને ઈનાસ્યોમેરિક સ્વરૂપમાં અસ્તિત્વમાં રહે તે પહેલાં કેટલા કાર્બન અણુઓની જરૂર છે?
- 18View Solutionઆલ્કેનના આયોડિનેશનમાં .......... નો ઉપયોગ જરૂરી છે.
- 19View Solutionઆલ્કેનના ક્લોરિનેશનની સાપેક્ષમાં બ્રોમિનેશન.......
- 20View Solutionઆલ્કેનનુ પ્રકાશરાસાયણિક ક્લોરિનેશન ..... દ્વારા શરૂ થાય છે.
- 21View Solutionઆલ્કેનનું હેલોજિનેશન એ કઇ ક્રિયાવિધિનું ઉદાહરણ છે ?
- 22View Solutionઆલ્કેેન માટે કયું વિધાન ખોટું છે.
- 23આલ્કોક્સિમર્ક્યુરેશન ડી-મર્ક્યુરેશન ની નીપજ $(A)$ ........છેView Solution
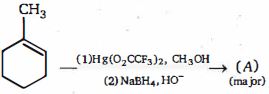
- 24View Solutionઆલ્કોહોલનું નિર્જલીકરણ ...... નું ઉદાહરણ છે.
- 25View Solutionઆશ્ચર્યજનક રીતે, નીચે આપેલી પ્રકિયા કાર્બોકેટાયન માંથી પસાર થાય છે. તો આ પ્રકિયા ની મુખ્ય નીપજ શું હશે ?
- 26View Solutionઇથાઇન એ....... માંથી મેળવી શકાય છે.
- 27View Solutionઇથાઇનની સમાનધર્મીં શ્રેણીમાં આવતું સંયોજન......... છે.
- 28ઇથાઇનને $HgSO_4$ અને મંદ $H_2SO_4$ ની હાજરીમાં $333\,K$ તાપમાને ગરમ કરતાં મળતી અંતિમ નીપજ......... છે.View Solution
- 29ઇથાઇલ આયોડાઇડની આલ્કોહોલિક $KOH$ સાથે પ્રક્રિયાથી ઉદભવતો વાયુ કે જે બેઝિક $KMnO_4$ નો રંગ દૂર કરે છે, તે........ છે.View Solution
- 30ઇથાઇલ આલ્કોહોલને લાલ ફોસ્ફરસ અને $HI$ સાથે ગરમ કરતા શું મળે છે ?View Solution
- 31ઇથાઇલ બેન્ઝિનના $KMnO_4$ વડે ઓક્સિડેશનથી કઇ નીપજ મળે છે ?View Solution
- 32ઇથાઇલ બેન્ઝિનનું $KMnO_4$ વડે ઓક્સિડેશન ...... સંયોજન બનાવે છે.View Solution
- 33ઇથાઇલ બ્રોમાઇડ આલ્કોહોલિક $KOH$ સાથે પ્રક્રિયા કરે છે અને..... આપે છે.View Solution
- 34ઇથાઇલ બ્રોમાઈડ ને અનુસરીને એક મોલ $1,2$ -ડાયબ્રોમોપ્રોપેન એ $NaNH_2$ ના $X$ મોલ્સ સાથે પ્રકિયા કરીને પેન્ટાઈન આપે છે તો $X$ ની કિમંત શોધોView Solution
- 35ઇથાઈલ આયોડાઇડ અને $ n- $ પ્રોપાઈલ આયોડાઇડને વુર્ત્જ પ્રક્રિયા પસાર કરવાની મંજૂરી છે. આ પ્રતિક્રિયામાં કયો આલ્કેન પ્રાપ્ત થશે નહીં ?View Solution
- 36View Solutionઇથિલીન, કાર્બન મોનોક્સાઇડ અને પાણીના મિશ્રણને ઊચા તાપમાને ગરમ કરતાં શુ મળે છે ?
- 37View Solutionઇથીન અણુનો આકાર....... છે.
- 38ઇથીનની $Br_2$ સાથેની યોગશીલ પ્રક્રિયાની નીપજ...... છે.View Solution
- 39View Solutionઇથીલીન અને એસિટિલીન વચ્ચે ફરક પારખવા માટે નીચેનામાંથી કોનો ઉપયોગ થાય છે?
- 40ઇથેન $(I)$, ઇથીન $(II)$, ઇથાઇન $(III$) અને પ્રોપિન $(IV)$ ના એસિડિક ગુણધર્મ માટે ઉતરતો ક્રમ કયો છે ?View Solution
- 41View Solutionઇથેન દહન પ્રક્રિયાને આધિન છે. દહન દરમિયાન કાર્બનનું સંકરણ ક્યાથી ક્યાં બદલાય છે ?
- 42View Solutionઇન્ડેન .............. છે.
- 43View Solutionઇલેકટ્રોનની વિસ્થાપનના નીચેનામાંથી કઇ પ્રક્રિયા બેન્ઝિન કરતાં ધીમી છે ?
- 44View Solutionઇલેક્ટ્રોન અનુરાગી યોગશીલ પ્રક્રિયા તરફ અણુઓની પ્રક્રિયાના દરમાં ઘટાડાનો ક્રમ કયો છે?
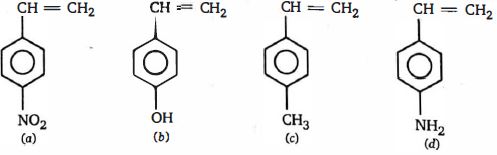
- 45ઇલેક્ટ્રોન અનુરાગી વિસ્થાપન પ્રક્રિયામાં $(I)$ એનિલિન, $(II)$ બેન્ઝિન તથા $(III)$ નાઇટ્રોબેઝિનની સક્રિયતાનો સાચો ક્રમ ..... છે.View Solution
- 46View Solutionઈથાઈલ એસિટેટનું પાયરોલીસીસ શું આપે છે ?
- 47ઈથાઈલ બેન્ઝિનનું $Cl_2$ સાથે મોનોક્લોરીનેશન કરવાથી ઉષ્મા ઉદભવતા શું મળે છે ?View Solution
- 48ઈથાઈલ બેન્ઝિનનું $KMnO_4$ દ્વારા ઓક્સિડેશન કરતા કયો પદાર્થ મળે છે ?View Solution
- 49ઈથીનના $\pi$-બંધમાં નોડલ પ્લેન કયુ આવેલું હોય છે ?View Solution
- 50ઈથીલીન એ $Br_2$ સાથે પ્રક્રિયા કરે છે. $1, 2$ ડાયબ્રોમો ઈથેન આપે છે. કયા મધ્યવર્તીંના નિર્માણના કારણે પ્રતિ (એન્ટી) યોગશીલ થાય છે ?View Solution
- 51ઉદીપક $P$ એ $Q$ અથવા $R$ નીપજ આપે છેView Solution
સંભવિત ઉદીપક :
$(I)\, 2Na/liq.NH_3$ $(II)\, H_2 /Pd/CaCO_3$ (ક્વિનોલાઇન) $(III)\, 2H_2 / Pd /C$
ઉપરોક્ત નીપજને ધ્યાનમાં રાખીને સાચું વિધાન કયું છે ?
- 52View Solutionઉદીપકીય હાઇડ્રોજનના કેટલા આલ્કિન ઉત્પાદન તરીકે આઇસોપેન્ટેન આપવામાં આવે છે
- 53ઉદીપકીય હાયડ્રોજીનેશન માં કયો આલ્કાઇન એ $3$ -ઇથાઇલહેકઝેન આપે છે ?View Solution
- 54View Solutionઉપર ની પ્રકિયા માં કેટલી ડાયક્લોરો ની નીપજ મળશે (અવકાશીય સમઘટ્કતા ને ઉમેરતા)
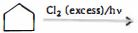
- 55View Solutionઉપર ની પ્રકિયા માં કેટલી નીપજ મળશે ?
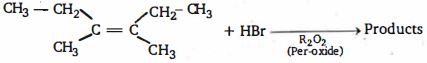
- 56ઉપર ની પ્રકિયા માં નીપજ $(K)$ શું હશે ?View Solution
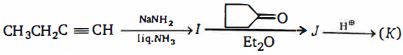
- 57View Solutionઉપરોક્ત આપેલી વુર્ત્જ પ્રક્રિયામાં પ્રાપ્ત નીપજ કઈ છે ?
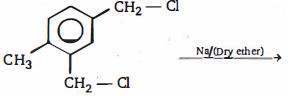
- 58ઉપરોક્ત પ્રકિયા ની નીપજ $(A)$ શું હશે ?View Solution
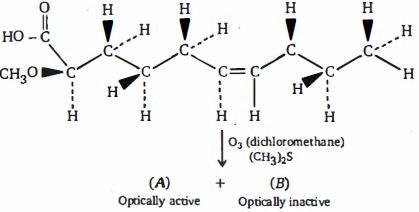
- 59View Solutionઉપરોક્ત પ્રકિયા માં છેલી નીપજ કઈ મળશે ?
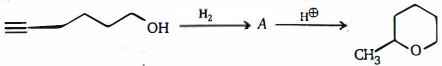
- 60View Solutionઉપરોક્ત પ્રકિયા માટે સાચું વિધાન કયું છે ?
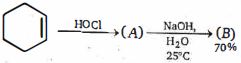
- 61ઉપરોક્ત પ્રકિયા માં નિપજ $(B)$ શું છે ?View Solution
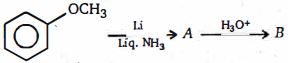
- 62ઉપરોક્ત પ્રકિયા માં નીપજ $(A)$ શું હશે ?View Solution
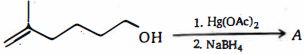
- 63ઉપરોક્ત પ્રકિયા માં નીપજ $(B)$ શું છે ?View Solution

- 64ઉપરોક્ત પ્રકિયા માં નીપજ $(C)$ ?View Solution
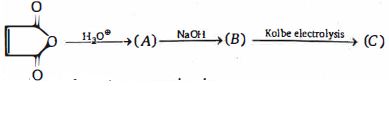
- 65ઉપરોક્ત પ્રકિયા માં નીપજ $(C)$ શું હશે ?View Solution
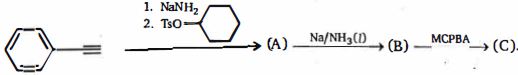
- 66ઉપરોક્ત પ્રકિયામાં પ્રકિયક $(A)$ અને $(B)$ શું હશે ?View Solution
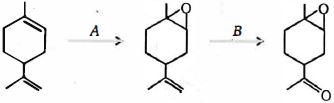
- 67View Solutionઉપરોક્ત પ્રક્રિયાની નીપજ શું હશે ?
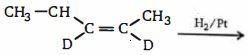
- 68ઉપરોક્ત પ્રક્રિયામાં ગ્લાયોક્સેલનું પાયરુંઆલ્ડિહાઈડ નું પ્રમાણ શું છે? ?View Solution
(figure) $\xrightarrow[{(2)\,Zn}]{{(1)\,{O_3}}}\mathop {\begin{array}{*{20}{c}}
{\,\,\,\,O\,\,\,\,\,\,\,O\,\,} \\
{\,||\,\,\,\,\,\,\,\,\,||} \\
{H - C - C - H}
\end{array}}\limits_{Glyoxal} + $ $\mathop {\begin{array}{*{20}{c}}
{\,\,\,O\,\,\,\,\,O\,\,} \\
{\,||\,\,\,\,\,\,\,\,||} \\
{C{H_3} - C - C - C{H_3}}
\end{array}}\limits_{2,3 - Bu\tan edione} + \mathop {\begin{array}{*{20}{c}}
{\,\,\,\,\,\,O\,\,\,\,\,O} \\
{\,\,\,\,\,\,||\,\,\,\,\,\,||} \\
{C{H_3} - C - C - H}
\end{array}}\limits_{Pyrualdehyde} $
- 69ઉપરોક્ત રૂપાંતર ને બનાવવા માટે $(A)$ અને $(B)$ શું હશે ?View Solution
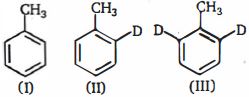
- 70ઉપરોક્ત સંયોજનોના $o-$ નાઈટ્રેશનનો દર, $(I)$ ટોલ્યુઇન, $(II)\, 2-D-$ ટોલ્યુઇન અને $(III) \,2, 6-D_2$ --ટોલ્યુઇન છે નીચેના ક્રમમાં કયો છેView Solution
- 71View Solution.......... ઉમેરવાથી ડીઝલનો ઓકટેન આંક વધે છે.
- 72View Solutionઍસિડિક હાઇડ્રોજન .... માં રહેલો છે.
- 73View Solution....... એ આલ્કેનનું ઉદાહરણ છે.
- 74View Solutionએકથી વધુ પ્રકારના સંકરણ ધરાવતા કાર્બન જેમાં હોય તેવું સંયોજન..... છે.
- 75એક બળતણ $70\%$ આઇસોઓક્ટેન તથા $30\%$ $n-$ હેપ્ટેન - ના મિશ્રણ જેટલો જ knocking ગુણ ધરાવે છે. તો બળતણનો ઓક્ટેન આંક ............. થશે.View Solution
- 76એક મોલ સંમિતિય આલ્કીન પર ઓઝોનોલીસીસ કરતા બે મોલ આલ્ડીહાઈડ જે અણુમાં $44 u$ ધરાવતા પદાર્થ આવે છે તો આલ્કીન કયો હોય ?View Solution
- 77એક હાઇડ્રોકાર્બન $A (V.D. = 36)$ ફક્ત એક જ મોનોક્લોરો વિસ્થાપન ઉત્પાદન બનાવે છે. $A$ શું હશે ?View Solution
- 78એક હાઇડ્રોકાર્બનની હાઇપોક્લોરસ એસિડ સાથેની પ્રક્રિયા $2-$ ક્લોરોઇથેનોલ આપે છે. તો તે હાઇડ્રોકાર્બન ......... હશે.View Solution
- 79View Solutionએરોમેટિક કેન્દ્રના ક્લોરિનેશનમાં ક્યો ઘટક ઉત્પન્ન થાય છે ?
- 80View Solutionએરોમેટિક સંયોજનના નાઈટ્રેશન પરથી નીચેનામાંથી કયુ વિધાન સાચું છે ?
- 81View Solutionએરોમેટીક ચક્ર ઉપર પ્રબળ નિષ્ક્રિય અસર કોના દ્વારા થાય ?
- 82એસિટિલિન મંદ $H_2SO_4$ સાથે પ્રક્રિયા કરી ....... નીપજાવે છે.View Solution
- 83View Solutionએસિટિલિન ..... સાથે પ્રક્રિયા કરતું નથી.
- 84View Solutionએસિટિલિન હાયપોક્લોરસ ઍસિડ સાથે પ્રક્રિયા કરે ત્યારે શું બને ?
- 85એસિટિલીનને મરક્યુરિક સલ્ફેટ ધરાવતા મંદ સલ્ફયુરિક એસિડના દ્રાવણમાંથી પસાર કરતાં મળતી અંતિમ નીપજમાં $\pi$ બંધની સંખ્યા......... છે.View Solution
- 86એસિટીલીન એ આલ્કલાઈન $KMnO_4$ સાથે પ્રક્રિયા કરીને શું બનાવે છે ?View Solution
- 87View Solutionએસિટીલીન એ હાઈપોક્લોરસ એસિડ સાથે પ્રક્રિયા કરે તો નીપજ શું હશે ?
- 88View Solutionએસિટોન એ......ના ઓઝોનોલીસીસથી બને છે.
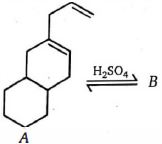
- 89એસિડના નિશાન ઉમેરવા પર $A$ આઇસોમેરાઇઝ $H_2SO_4$ .તો સંયોજન $(B)$ શું થશે.?View Solution
- 90એસીટીલીનને $Hg^{2+}$ આયનો ધરાવતા મંદ સલ્ફયુરીક એસીડ માંથી પસાર કરવામાં આવે તો કઇ નીપજ મળે છે ?View Solution
- 91ઑઝોનોલિસિસ પર $C_4H_6$ કાર્બનિક સંયોજન એ ,$HCHO,CO_2, CH_3CHO$. આપે છે. તો તેView Solution
સંયોજન કયું હશે ?
- 92View Solutionઓક્ટેન આંક કઇ પેટ્રોલિયમ પેદાશ સાથે સંકળાયેલો છે ?
- 93View Solutionઓક્ટેન આંક ................ દ્વારા બદલી શકાય છે.
- 94ઓર્થો નાઈટ્રોફિનોલ એ $p -$ અને $m -$ નાઈટ્રોફિનોલ કરતા પાણીમાં ઓછો દ્રાવ્ય છે કારણ કે....View Solution
- 95............ ઓલેફિનના ઓઝોનોલિસિસથી $CH_3CH_2CHO$ અને $CH_3CHO$ મળે છે.View Solution
- 96View Solutionઓલેફિન બંધમાં મિનરલ એસિડનો સમાવેશ મુખ્ય નીપજ તરફ લઈ જાય છે, તેને ઓળખો
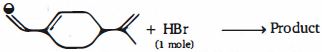
- 97View Solutionઓલેફિન્સ કોના દ્વારા હાઇડ્રોજીનેટેડ થઈ શકે છે ?
- 98કઇ પ્રકિયા $CH_2 = C = CH_2$ આપશે ?View Solution
- 99View Solutionકઇ પ્રક્રિયાથી પ્રોપેન બનતું નથી ?
- 100View Solutionકઇ પ્રક્રિયામાં આલ્કેન અને આલ્કીન બંને મળે છે ?