Question Bank
Explore our large set of questions to practice for your standard seamlessly- 1કઈ પ્રકિયા માં અંતિમ નીપજ $Ph -C \equiv CH$ હશે ?View Solution
- 2કઈ પ્રક્રિયા દ્વારા $1$-બ્યુટીને એ બ્યુટેનમાં રૂપાંતર પામે છે ?View Solution
- 3View Solutionકઈ પ્રક્રિયામાં નીપજ કીરાલ કેન્દ્રના પરિણામે જોવા મળે છે ?
- 4કઈ પ્રક્રીયામાં નીપજ, $2, 2$-ડાયબ્રોમોપ્રોપેન મળે છે ?View Solution
- 5કઈ પ્રતિક્રિયામાં સૌથી ઓછુ $\Delta {{G}^{\begin{smallmatrix}View Solution
+ \\
+
\end{smallmatrix}}}$ અથવા (સક્રિયકરણ ઉર્જા) - 6કયા નિયમને આધારે $2$-બ્યુટેનોલના ડિહાઇડ્રેશનથી $2$-બ્યુટીન મુખ્ય નીપજ તરીકે મળે છે ?View Solution
- 7કયા પદાર્થ એમોનિકલ $AgNO_3$ ના દ્રાવણ સાથે પ્રક્રીયા કરવા સક્ષમ છે ?View Solution
- 8કયા પ્રક્રિયક દ્વારા $1$-બ્યુટાઈન અને $2$-બ્યુટાઈન વચ્ચે ભેદ પારખી શકાય છે ?View Solution
- 9View Solutionકયામાં પ્રતિ મોલ સૌથી નીચી હાઈડ્રોજીનેશન ઉષ્મા ધરાવે છે ?
- 10View Solutionકયાં સંયોજનો યોગશીલ પ્રક્રિયા ન દર્શાવે ?
- 11View Solutionકયું એમોનિકલ સિલ્વર નાઈટ્રેટના દ્વાવણ સાથે પ્રક્રિયા કરતું નથી ?
- 12View Solutionકયુ એસિટીલીન સાથે પ્રક્રિયા કરતું નથી ?
- 13કયુ સંયોજન એ $Br_2$ થી $1$ -બ્યુટીનના ઉમેરાથી સંભવિત નીપજ છે?View Solution
- 14View Solutionકયું સંયોજન પાણી સાથેની પ્રક્રિયાથી મિથેન વાયુ આપે છે ?
- 15View Solutionકયું સંયોજન વિસ્થાનીકૃત ઇલેક્ટ્રોન ધરાવે છે ?
- 16View Solutionકયુ સંયોજન વિસ્ફોટક તરીકે વપરાય છે ?
- 17View Solutionકયો અણુ દ્વિ - ધ્રુવ ચાકમાત્રા ધરાવે છે ?
- 18કયો આલ્કીન ઉદ્દીપકીય હાઈડ્રોજીનેશન પરિસ્થિતિમાં $H_2$ સાથે ઝડપથી પ્રક્રિયા કરે છે ?View Solution
- 19View Solutionકયો આલ્કેન માત્ર એક મોનો ક્લોરીનેટેડ નીપજ આપે છે ?
- 20View Solutionકાર્બન પદાર્થનું ઓઝોનોલીસીસ એ એક નીપજ તરીકે ફોર્માલ્ડીહાઈડ આપે છે જે.......ની હાજરી દર્શાવે છે.
- 21View Solutionકાર્બનિક સંયોજનોમાં રહેલી અસંતૃપ્તતા ........... દ્વારા પારખવામાં આવે છે.
- 22કાલ્પનિક$1, 3,5-$ સાયકલોહેકઝેટેરિન ની સાથે બેન્ઝિનના હાઇડ્રોજનને સરખામણી કરતી વખતે, બેન્ઝિન .................... સાયક્લોહેક્સેટ્રેન કરતાં.View Solution
- 23View Solutionકુદરતી વાયુ ....... નુ મિશ્રણ છે.
- 24View Solutionકુદરતી વાયુ .........નું મિશ્રણ છે?
- 25કેલિન $C_7 H_6$ , એકદમ ધ્રુવીય ચક્રીય પરમાણુ હોવાની અપેક્ષા છે. નીચેનામાંથી ક્યા સંસ્પંદન સ્વરૂપો પરમાણુની વાસ્તવિક રચના (સંસ્પંદન સંકર) ને લગાવવામાં સૌથી મોટી મર્યાદામાં ફાળો આપે છેView Solution
- 26View Solutionકેલ્શિયમ કાર્બાઇડની પાણી સાથેની પ્રક્રિયાથી....... મળે છે.
- 27કેલ્શિયમ કાર્બાઈડ + ભારે પાણી $ \to$ ?View Solution
ઉપર ની પ્રકિયા માં નીપજ શું હશે ? - 28View Solutionકોલસાની ખાણમાં વિસ્ફોટ માટે જવાબદાર વાયુ ....... છે.
- 29View Solutionક્યા પ્રક્રિયકોની શ્રેણી દ્વારા એસિટિલિનનું ઉચ્ચ આલ્કાઇનમાં રૂપાંતર કરી શકાય છે ?
- 30View Solutionક્યુબેન ના સમાન દ્વિ બંધ કેટલા છે .
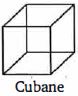
- 31View Solutionક્લોરોપ્રિન બનાવવા માટે એસિટિલિનનુ ડાયમરાઈઝેશન કરવા જરૂરી ઉદ્દીપક .... છે.
- 32View Solutionઘાત્વીય કાર્બાઇડની પાણી સાથેની પ્રક્રિયાથી ઉદભવતો રંગહીન વાયુ..... છે.
- 33View Solutionજંતુનાશક ગેમેક્જિન = .......
- 34View Solutionજયારે બળતણ મુખ્યત્વે ............... ધરાવે ત્યારે એન્જિનમાં knocking વધારે થાય છે.
- 35View Solutionજે આલ્કિન ભૌમિતિક સમઘટકતા દર્શાવે છે તે ...... છે.
- 36View Solutionજો ઓઝોનોલીસીસની નીપજ એસિટોન અને ફોર્માલ્ડીહાઈડ હોય તો પ્રારંભિક પદાર્થ શું હશે ?
- 37જ્યારે $1$ -methylcyclohexene અને $NBS$ વચ્ચેની પ્રક્રિયા જલીય ડાયમીથાઇલ સલ્ફોક્સાઇડમાં આપે છે ત્યારે નીચેનામાંથી કયું શ્રેષ્ઠ અવકાશીય રસાયણિકરીતે રજૂ થાય છે?View Solution
- 38જ્યારે $1$-બ્યુટીન ને $Al_2(SO_4)_3$ સાથે $500\,K$ ગરમ કરતા કઈ નીપજ બને છે ?View Solution
- 39જ્યારે $CH_2 = CH(CH_2)2COOH$ ની $HBr$ સાથે પ્રક્રિયા કરે છે ત્યારે નીચેનામાંથી કયું સંયોજન બનાવે છે ?View Solution
- 40જ્યારે $CH_3CH_2CHCl_2$ ને પ્રક્રિયા $NaNH_2$ સાથે કરવામાં આવે તો નીપજ કઈ મળે છે ?View Solution
- 41જ્યારે $FeBr_3$ ની હાજરીમાં નાઇટ્રોબેન્ઝિન $Br_2$ સાથે પ્રક્રિયા કરવામાં આવે તો મુખ્ય નિપજ તરીકે બને એ.....View Solution
- 42જ્યારે $H_2SO_4$ ની હાજરીમાં ફિનાઇલ એસિટિલીન મંદ $HgSO_4$ સાથે પ્રક્રિયા કરે તો શું મળે છે ?View Solution
- 43જ્યારે $HgCl_2$ ની હાજરીમાં એસિટિલીનની પ્રક્રિયા $HCl$ સાથે થાય ત્યારે મળતી નીપજ......... છે.View Solution
- 44જ્યારે $2$ -બ્યુટાઈન એ $Pd - BaSO_4$ સાથે પ્રકિયા કરે છે ત્યારે નીપજ કઈ મળે છે ?View Solution
- 45જ્યારે આલ્કોહોલીક $KOH$ ને $CH_3CH_2Cl$ સાથે પ્રક્રિયા કરતા આલ્કીનનું નિર્માણ થાય તો પ્રક્રિયામાં હુમલો કરતો ઘટક કયો છે ?View Solution
- 46જ્યારે ઉપરના સંયોજનમાં ઉત્પ્રેરક હાઇડ્રોજન છે? ત્યારે કયુ ($\pi$ -bond) પ્રથમ ઘટાડશે,View Solution
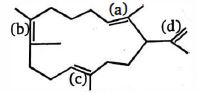
- 47જ્યારે એસિટિલિનને મંદ $H_2SO_4$ માંથી $HgSO_4$ ની હાજરીમાંથી પસાર કરતાં બનતું સંયોજન ..... છે.View Solution
- 48જ્યારે ઓક્સિડેટીવ ઓઝોનોલિસિસમાંથી પસાર થાય છે ત્યારે કયું સંયોજન એ $CO_2$ ગેસ મુક્ત કરતો નથી ?View Solution
- 49View Solutionજ્યારે ટોલ્યુઈનને પ્રકાશની હાજરીમાં અને હેલોજન કેરીયરની ગેરહાજરીમાં ક્લોરીન સાથે ગરમ કરવામાં આવે તો શું ઉત્પન્ન થાય ?
- 50View Solutionજ્યારે નિર્જળ એલ્યુમિનિયમ ક્લોરાઇડની હાજરીમાં બેન્ઝિનની ઇથાઇલક્લોરાઇડ સાથે પ્રક્રિયા કરવામાં આવે તો કયું સંયોજન મળે ?
- 51જ્યારે બેન્ઝિન સાંદ્ર $HCl$ સાથે ઉકાળવામાં આવે તો કયું સંયોજન મળે છે ?View Solution
- 52જ્યારે વધુ $C_6H_6$ ને નિર્જળ $AlCl_3$ ની હાજરીમાં $CH_2Cl_2$ સાથે પ્રક્રિયા કરતા નીચેનામાંથી કયું બંધારણ નિપજ ધરાવતું મળશે ?View Solution
- 53View Solutionઝાયલીનનો કયો સમઘટક ત્રણ જુદા જુદા મોનોક્લોરો વ્યુત્તપણ આપી શકે છે
- 54View Solutionટેટ્રાબ્રોમો ઇથેનની ઝીંક સાથેની પ્રક્રિયાથી ............. મળે છે.
- 55View Solutionટોલ્યુઇન અને ક્રોમાઇલ ક્લોરાઇડ પ્રક્રિયા કરી..... બનાવે છે.
- 56View Solutionટોલ્યુઇનના નાઇટ્રેશનથી અંતિમ નિપજ .......
- 57View Solutionટોલ્યુઇનના સંપૂર્ણ નાઇટ્રેશનથી મળતી નીપજ......... છે.
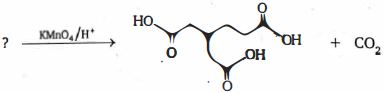
- 58ટોલ્યુઇનની $KMnO_4$ સાથેની પ્રક્રિયાથી શું મળે છે ?View Solution
- 59ટોલ્યુઇનની એસિડિક $KMnO_4$ સાથેની પ્રક્રિયાથી ..........મળે છેView Solution
- 60View Solutionટોલ્યુઇનની ફેરિક ક્લોરાઇડની હાજરીમાં ક્લોરિન સાથેની પ્રક્રિયાથી મળતી નીપજ...... છે.
- 61ટોલ્યુઇનનુ $V_2O_5$ ની હાજરીમાં હવા સાથે ઓક્સિડેશન કરતા ........... મળે છે.View Solution
- 62ટોલ્યુઇનનુ મંદ $HNO_3$ વડે ઓક્સિડેશન ........... આપે છે.View Solution
- 63ટોલ્યુઇનમાં $(\pm)-2$-ક્લોરો-$2$-ફિનાઇલ ઇથેનના દ્રાવણને $SbCl_5$, ની ઓછા પ્રમાણની હાજરીમાં ધીમે ધીમેથી મિશ્ર કરવામાં આવે છે......ના નિર્માણને કારણે......View Solution
- 64View Solutionટોલ્યુઈનનું.......સાથેના ઓક્સિડેશન કરવાથી બેન્ઝાલ્ડિહાઇડમાં રૂપાંતર થાય છે.
- 65ટ્રાન્સ$-2$-ફીનાઈલ-$1$-બ્રોમોસાયક્લો પેન્ટેનની આલ્કોહોલીક $KOH$ સાથે પ્રક્રીયા કરતા શું મળે છે ?View Solution
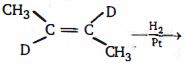
- 66ટ્રાન્સ-$2$-બ્યુટીન નીકલ ઉદ્દીપકની હાજરીમાં સાથે પ્રક્રિયા કરતા નીપજ શું મળે છે ?View Solution
- 67ટ્રાન્સ - સાયકલોહેકઝેન $1,2$ -ડાયોલ એ સાયકલોહેકઝીન ની કોની સાથે ની પ્રકિયા થી મેળવી શકાય છે ?View Solution
- 68ઠંડો આલ્કલાઇન $KMnO_4$ સાથે $2$ -બ્યુટીન ની પ્રક્રિયાની મુખ્ય નીપજ કઈ છે ?View Solution
- 69ડાય - ઈમાઈડ $(N_2H_4)$ નો ઉપયોગ કોના દ્વિ-બંધ ઘટાડવા માટે થાય છે ?View Solution
- 70તબ્બકાનો ક્રમ પસંદ કરો જે ઇથેનોલમાંથી $1-$ બ્યુટીનના શ્રેષ્ઠ સંશ્લેષણનું વર્ણન કરે છેView Solution
- 71ત્રણ સંતૃપ્ત હાઇડ્રોકાર્બન $A, B$ અને $C$ ના ઉત્કલનબિદુ અનુક્રમે $-102\,^oC, -43. 44\,^oC$ અને $-0.6\,^oC$ છે. અણુમાં સૌથી વધારે કાર્બન પરમાણુઓ હોય તેવો હાઇડ્રોકાર્બન ....... છે.View Solution
- 72View Solutionદહન ચેમ્બરમાં લેડ જમા થતું અટકાવવા પેટ્રોલમાં ઉમેરવામાં આવતુ રસાયણ ............... છે.
- 73View Solutionનાઇટ્રેશન મિશ્રણમાં સાંદ્ર સલ્ફયુરિક એસિડ ........... તરીકે વપરાય છે.
- 74View Solutionનાઇટ્રોબેન્ઝિન ના નાઇટ્રેશનથી ..... બને છે.
- 75View Solutionનાઇટ્રોબેન્ઝિનના નાઇટ્રેશનથી મળથી નીપજ..... છે.
- 76View Solution.......... ના જલીય દ્રાવણના વિદ્યુતવિભાજનથી ઇથિલિન મળે છે.
- 77View Solution......... ના વિધુતવિભાજનથી બેન્ઝિન મળે છે.
- 78નિપજ $(B)$ એ શું હશે ?View Solution
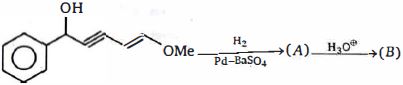
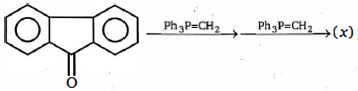
- 79નિપજ $(x)$ એ..........View Solution
- 80View Solutionનિપજ એ શું મળે છે ?
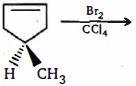
- 81View Solutionનિપજ શું હશે ?
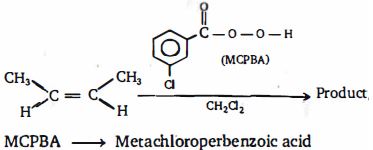
- 82નિપજો $P$ અને $Q$ નીચેની પ્રક્રિયાઓના કયા ક્રમમાં છે ?View Solution
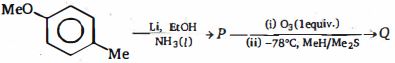
- 83View Solutionનિપજો ની પ્રકાશક્રિયાશીલ પર વિધાન કરો
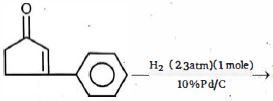
- 84નીચા તાપમાને ($0°$ સે. અથવા નીચે) $1,3$ બ્યુટાઈનમાં $1$ મોલ $HCl$ ઉમેરતા કઈ મુખ્ય નીપજ મળશે ?View Solution
- 85View Solutionનીચે આપેલ કઈ પ્રકિયા એવી છે કે જેની નીપજ રેસેમિક મિશ્રણ છે ?
- 86નીચે આપેલ પ્રક્રિયામાં રચાયેલ મુખ્ય કાર્બનિક નીપજ કઈ છે?View Solution
$C{H_2} = CH{(C{H_2})_8}COOH + HBr\xrightarrow{{peroxide}}....$
- 87View Solutionનીચે આપેલા પરમાણુઓના હાયડ્રોજીનેશનની ઉષ્માનો યોગ્ય ક્રમ કયો છે ?
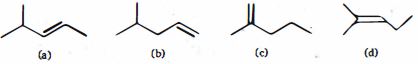
- 88નીચે આપેલા મુક્ત મુલક માં બ્રોમિનેશન પ્રક્રિયાઓ વચ્ચે, તે પસંદ કરો જેમાં $2^o$ હેલાઈડ મુખ્ય નીપજ છેView Solution
- 89નીચે આપેલા સંયોજન $(3)$ ને બાકીના સંયોજનોથી અલગ પાડવા માટે સૌથી યોગ્ય પ્રક્રીયક ક્યો છે ?View Solution
$(1)$ $CH_3 - C \equiv C - CH_3$ $(2)$ $CH_3CH_2CH_2CH_3$
$(3)$ $CH_3CH_2C \equiv CH$ $(4)$ $CH_3CH = CH_2$
- 90View Solutionનીચે આપેલા હાયડ્રોજીનેશન ની પસંદગી ની પ્રકિયા માં કયું મિશ્રણ ખોટું છે ?
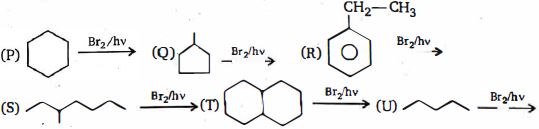
- 91View Solutionનીચે આપેલી ઓક્સિડેશન માટે કયું સંયોજન એ પ્રારંભિક સામગ્રી હતી?
- 92View Solutionનીચે આપેલી પ્રકિયા ની નીપજ શું હશે ?
- 93View Solutionનીચે આપેલી પ્રકિયા ની મુખ્ય નીપજ કઈ હશે ?
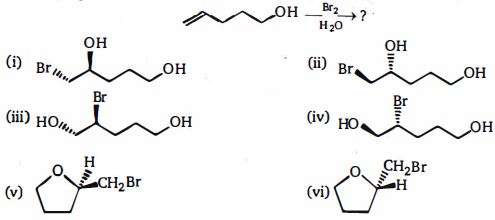
- 94View Solutionનીચે આપેલી પ્રકિયામાંથી કયા બ્રોમાઇડ્સની મુખ્ય નીપજ છે ,એવું માનીને કે કોઈ કારબોકેટાયન ફરીથી ગોઠવાતો નથી?
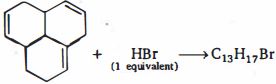
- 95નીચે આપેલી પ્રકિયા માં નીપજ શું હશે ?View Solution
$\begin{array}{*{20}{c}}
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,OH} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|} \\
{C{H_3}{\text{ }} - {\text{ }}CH{\text{ }} = {\text{ }}CH{\text{ }} - {\text{ }}C{H_3}\xrightarrow{{{H_3}{O^ + }}}\,C{H_3} - C{H_2} - CH - C{H_3}}
\end{array}$ - 96View Solutionનીચે આપેલી પ્રકિયા માં મુખ્ય નીપજ કઈ છે ?
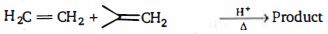
- 97View Solutionનીચે આપેલી પ્રકિયા માં મુખ્ય નીપજ કઈ છે ?
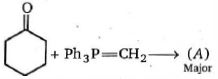
- 98View Solutionનીચે આપેલી પ્રકિયા માં મુખ્ય નીપજ કઈ હશે ?
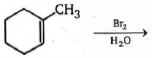
- 99View Solutionનીચે આપેલી પ્રકિયા માં મુખ્ય નીપજ બને છે કઈ છે ?
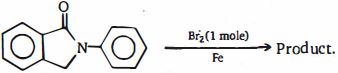
- 100નીચેના આલકેન્સને તેમના દહન ઉષ્મા ના ઘટતા ક્રમમાં ગોઠવો.View Solution
$(i)\,\mathop {\begin{array}{*{20}{c}}
{\,\,\,\,\,C{H_3}} \\
| \\
{C{H_3} - C - C{H_3}} \\
| \\
{\,\,\,\,\,C{H_3}}
\end{array}}\limits_{(Neo - pen\tan e)\,(i)} $$(ii\mathop {)\,\begin{array}{*{20}{c}}
{C{H_3} - CH - C{H_2} - C{H_3}} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{\,\,\,\,\,C{H_{3\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}
\end{array}}\limits_{(Iso - pen\tan e)\,\,(ii)} $$(iii)\,\mathop {C{H_3} - C{H_2} - C{H_2} - C{H_2} - C{H_3}}\limits_{(n - pen\tan e)} $