$0.1\,M\,KCl$ ના દ્રાવણમાં $AgCl\,\,(K_{sp} = 1.0 \times 10^{-10}$) ની દ્રાવ્યતા ( $mol\,L^{-1}$ માં ) કેટલી થશે ?
AIEEE 2012, Diffcult
a
Let solubility of \(\bar AgCl = x\,mole/L\)
Let solubility of \(\bar AgCl = x\,mole/L\)
\(AgCl \rightleftharpoons A{g^ + } + C{l^ - }\)
i.e., \({K_{sp(AgCl)}} = x \times x\)
\(KCl \to {K^ + } + \mathop {C{l^ - }}\limits_{0.1} \)
\([C{l^ - }]\,\) from \(KCl = 0.1\,m\)
Total \([C{l^ - }]\) in solution \( = x + 0.1\)
\({K_{sp}}(AgCl) = [A{g^ + }][C{l^ - }] = x(x + 0.1)\)
\(1.0 \times {10^{ - 10}} = x(x + 0.1)\)
\(1.0 \times {10^{ - 10}} = {x^2} + 0.1x\)
\(1.0 \times {10^{ - 10}} = 0.1x\) (as \({x^2} < < 1\))
\(x = 1.0 \times {10^{ - 9}}\,mol/L\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1એક બેઈઝને પાણીમાં દ્રાવ્ય કરતા હાઇડ્રોક્સિલ આયનની સાંદ્રતા $0.05$ મોલ લિટર$^{-1}$ ધરાવતું દ્રાવણ આપે તો તે દ્રાવણ .... થશે.View Solution
- 2View Solutionનીચેના સંતુલન વિશે કયું વિધાન સાચું છે
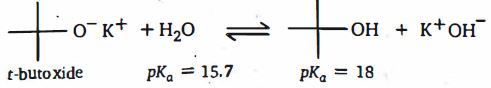
- 3$0.2\, M$ એમોનિયાના $50\, mL$ દ્રાવણને $0.2\, M\, HCl$ ના $25\, mL$ દ્રાવણમાં ઉમેરવામાં આવે છે. જો એમોનિયાના દ્રાવણનો $pK_b$ $4.75$ હોય, તો મિશ્રણની $pH$ જણાવો.View Solution
- 4View Solutionકયું બફર તરીકે કાર્ય કરે છે ?
- 5$10^{-2}\, M\, HCN$ અને $[H^+]$ = $10^{-3}$ માટે વિયોજન અચણાંક નું મુલ્ય.....$\%$ માં શોધો.View Solution
- 6$H_3BO_3$ અને બોરેક્ષ દ્રાવણ ને..... કહે છે.View Solution
- 7$300\, cc \,0.3\, M \,NH_3$ અને $500\, cc\, 0.5\, M\, NH_4Cl$ ના મિશ્રણ દ્વારા બનાવેલ બફરની $pH$ શોધો $?$ $NH_3$ માટે $1.8 \times K_b = 10^{-5}\,\,K_b$.View Solution
- 8$0.001\,M $ એસિડીક એસિડની $pH$ $= .......$View Solution
- 9જો $25\,°C$ એ $MX_2$ ક્ષાર અલ્પ દ્રવ્યનો દ્રાવ્યતા ગુણાકાર $K_{sp} = 1.0 \times 10^{-11}$ છે. તો આ તાપમાને $L^{-1}$ મોલમાં ક્ષારની દ્રાવ્યતા $= ?$View Solution
- 10$Al_2(SO_4)_3 $ નુ જલીય દ્રાવણ = .......View Solution
