Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1એક વાયુ $1\,atm$. ના અચળ દબાણે $10\, dm^3$ માંથી $20\, dm^3$ કદમાં સમતાપી વિસ્તરણ પામે છે. જો તે પર્યાવરણમાંથી $800\, J$ ઉષ્મીય ઊર્જાનુ શોષણ કરે તો આ પ્રકમ માટે $\Delta U$ કેટલા .....$J$ થશે?View Solution
- 2View Solutionકેટલાક બરફના ટુકડા ધરાવતા ઉષ્મા પાત્રને સારી રીતે બંધ કરવામાં આવે જે શેનું ઉદાહરણ છે ?
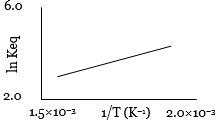
- 3એક પ્રક્રિયા માટે $\ln K_{eq}$ વિરુદ્ધ તાપમાનના વ્યસ્તનો આલેખ નીચે મુજબ છે. તો પ્રક્રિયા ........ હશે.View Solution
- 4જો $C(s),\,{H_2}(g)$ અને $C{H_4}(g)$ની દહન ઉષ્મા અનુક્રમે $ - 94,\, - 68$ અને $ - 213\,kcal/mol$ છે.View Solution
તો પ્રક્રિયા $C(s) + 2{H_2}(g)\, \to \,C{H_4}(g)$ માટે $(\Delta {H^o})$નું મૂલ્ય ........$kcal$ થશે.
- 5સાઇક્લોહેક્ઝિનની હાઇડ્રોજનીકરણ એન્થાલ્પીનું મૂલ્ય $-119.5\, kJ \,mol^{-1}$ છે. જો બેન્ઝિનની સંસ્પંદન ઊર્જા $-150.4\, kJ\,mol^{-1}$ હોય તો બેન્ઝિનની હાઇડ્રોજનીકરણ એનથાલ્પીનું મૂલ્ય ......$kJ\, mol^{-1}$ થશે ?View Solution
- 6$CO ({-1}10 \,KJ\, mol^{-1}$) અને $CO_2(-394 \,KJ$ મોલ$^{-1}$) ના નિર્માણની પ્રમાણિત એન્થાલ્પી છે. જ્યારે $1$ મોલ ગ્રેફાઈટ સળગે ત્યારે દહન ઉષ્મા કેટલા ............... $\mathrm{KJ}$ થશે ?View Solution
- 7View Solutionજ્યારે કોઈ વાયુ સમોષ્મી વિસ્તરણની અસર હેઠળ જાય છે ત્યારે તે ઠંડો થાય છે. કારણ કે....
- 8નીચેના પ્રકમો પરથી પ્રકિયા $B + D \rightarrow E + 2C$ માટે $\Delta H$ નું મૂલ્ય કેટલા ........... $\mathrm{kJ/mol}$ હશે તે જણાવો.View Solution
$\Delta H \,(kJ/mol)$ $\frac 12 A \rightarrow B$ $+150$ $3B \rightarrow 2C + D$ $-125$ $E + A \rightarrow 2D$ $+350$ - 9View Solutionબધા તાપમાને સ્વયંભૂ રહેવાની પ્રક્રિયા માટે
- 10$S.T.P.$ એ જળવાયુના $112$ લીટરના દહન દરમ્યાન મળતી ઉષ્મા કેટલા ......$KJ$ હશે ? ($H_2$ અને $CO$ ના સમાન કદનું મિશ્રણ)$H_2$$_{(g)} +$ $ \frac{1}{2} O_2$$_{(g)}$ $\rightarrow$ $H_2O$ $_{(g)}$; $\Delta H = -241.8 \,KJ; CO$ $_{(g)}$ $+ \frac{1}{2} O_2$ $_{(g)}$ $\rightarrow$ $CO_2$ $_{(g)}$; $\Delta H = -283\, KJ$View Solution
