[ આપેલ : પાણીનો મોલલ ઉત્કલનબિંદુ ઉન્નયન અચળાંક $\left(\mathrm{K}_{\mathrm{b}}\right)=0.52 \mathrm{~K} . \mathrm{kg} \mathrm{mol}^{-1}$,
$1 \mathrm{~atm}$ દબાણ $=760 \mathrm{~mm} \mathrm{Hg}$, પાણીનું મોલર દળ $\left.=18 \mathrm{~g} \mathrm{~mol}^{-1}\right]$
$ 2=0.52 \times \mathrm{m}$
$\mathrm{m}=\frac{2}{0.52}$
According to question, solution is much diluted
$\text { so } \frac{\Delta \mathrm{P}}{\mathrm{P}^{\circ}}=\frac{\mathrm{n}_{\text {solate }}}{\mathrm{n}_{\text {soltron: }}}$
$\frac{\Delta \mathrm{P}}{\mathrm{P}^{\circ}}=\frac{\mathrm{m}}{1000} \times \mathrm{M}_{\text {solvent }}$
$\Delta \mathrm{P}=\mathrm{P}^{\circ} \times \frac{\mathrm{m}}{1000} \times \mathrm{M}_{\text {sohtwat }}$
$=760 \times \frac{\frac{2}{0.52}}{1000} \times 18=52.615$
$P_5=760-52.615=707.385 \mathrm{~mm} \text { of } \mathrm{Hg}$
Download our appand get started for free
Similar Questions
- 1View Solutionકોને અદ્ય પારગમ્ય પડદામાંથી પસાર કરી શકાય છે?
- 2એક મોલલ વિદ્યુત વિભાજ્ય $A _{2} B _{3}$ નું જલીય દ્રાવણ $60\%$ આયનીકરણ પામેલ છે. તો $1\,atm$ પર, આ દ્રાવણનું ઉત્કલન બિંદુ .......... $K$ છે. (નજીકના પૂર્ણાકમાં રાઉન્ડ ઑફ)View Solution
[આપેલ $K_b (H_2O) = 0.52\, K\, kg\, mol^{-1}]$
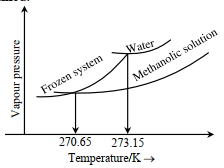
- 3જ્યારે'$x^{\prime} \times 10^{-2} \mathrm{~mL}$ મિથેનોલ (મોલર દળ=32 $\mathrm{g}$; ઘનતા $=0.792 \mathrm{~g} / \mathrm{cm}^3$ ) ને $100 \mathrm{~mL}$ પાણીમાં (ઘનતા $=1 \mathrm{~g} / \mathrm{cm}^3$ ), ઉમેરવામાં આવે છે ત્યારે નીચે મુજબ નો ડાયાગ્રામ પ્રાપ્ત થાય છે.View Solution
$(Image)$
$x=$.. . . . . .(નજીક નો પૂર્ણાક)
[આપેલ : $273.15 \mathrm{~K}$ પર પાણીનો મોલલ ઠારણ બિંદુ અવનયન અયળાંક $1.86 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}$ છે]
- 4$1$ મોલ નીચેના સંયોજનોમાંના દરેકને $1\,L$ દ્રાવણ માં ઓગળવામાં આવે છે.નીચેનામાંથી કોની $\Delta T_b$ ની કિંમત વધુ હશે ?View Solution
- 5View Solutionજ્યારે સામાન્ય ક્ષાર પાણીમાં ઓગળી જાય છે ત્યારે
- 6નીચેનામાંથી કોના $0.1\,M$ જલીય દ્રાવણનુ ઠારબિંદુ સૌથી નીચું હશે ?View Solution
- 7બે શુદ્ધ પ્રવાહીએ $(A) $ અને $(B) $ ના બાષ્પ દબાણ અનુક્રમે $100$ અને $80$ ટોર છે. જ્યારે $2 $ મોલ $(A)$ અને $3$ મોલ $ (B) $ ને મિશ્ર કરવાથી બનતા દ્રાવણનું કુલ દબાણ ......... ટોર થાય.View Solution
- 8માનવ રક્તનું સરેરાશ અભિસરણ દબાણ ${37\,^o}C$ તાપમાને $7.8$ બાર છે જલીય $NaCl$ દ્રાવણ ની સાંદ્રતા કેટલી છે જેનો ઉપયોગ રક્ત પ્રવાહમાં થઈ શકે છે ..........$mol/L$View Solution
- 9$0.5\, g$ એન્થ્રાસીનને $35\, g$ ક્લોરોફોર્મમાં દ્રાવ્ય કરતા ઉત્કલનબિંદુમાં $0.3\, K$ નો વધારો થાય છે. જો $CHCl_3$ માટે $K_b$ નુ મૂલ્ય $3.9\,K\,m^{-1}$ હોય, તો એન્થ્રાસીનનુ પ્રાયોગિક આણ્વિય દળ ......... $\mathrm{g\,mol}^{-1}$ થશે.View Solution
- 10વૉન્ટ હોફ અવયવ $'i'$ ધરાવતા નિર્બળ બેઇઝ $BOH$ ના $C\,M$ દ્રાવણ માટે બેઇઝ વિયોજન અચળાંક ........... થશે.View Solution
