$ 2 \mathrm{H}_{\text {(aq.) }}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_2(\mathrm{~g}) $
$ \mathrm{E}_{\text {cell }}=\mathrm{E}_{\text {cell }}^0-\frac{0.059}{2} \log \frac{\mathrm{P}_{\mathrm{H}_2}}{\left[\mathrm{H}^{+}\right]^2} $
$ =0-0.059 \times 3=-0.177 \text { volts. }=-17.7 \times 10^{-2} \mathrm{~V} .$
Download our appand get started for free
Similar Questions
- 1સેલ $Zn/Z{n^{2 + }}\,(1M)\,||\,C{u^{2 + }}\,(1M)/Cu$ પ્રમાણિત કોષ પોટેન્શિયલ શું છે? ${E^o}$ ના મૂલ્યો $Zn/Z{n^{2 + }}(1M) = - 0.76\,V$ અને $C{u^{2 + }}/Cu = + 0.34\,V$ છે.View Solution
- 2$NaCl,\,HCl$ તથા $C{H_3}COONa$ ની અનંત મંદને તુલ્યવાહકતા અનુક્રમે $126.45,\,426.16 $ $91\,oh{m^{ - 1}}\,c{m^2}\,mo{l^{ - 1}}$ હોય તો $C{H_3}COOH$ ની અનંત મદને તુલ્યવાહકતા .............. ${\Omega ^{ - 1}}{\rm{c}}{{\rm{m}}^2}{\rm{mo}}{{\rm{l}}^{ - 1}}$View Solution
- 3આપેલ કોષ માટે: $Cu ( s )\left| Cu ^{2+}\left( C _{1} M \right) \| Cu ^{2+}\left( C _{2} M \right)\right| Cu ( s )$ .જો ગિબ્સ ઊર્જામાં ફેરફાર $(\Delta G )$ ઋણ છે તો,View Solution
- 4$298 \mathrm{~K}$. પર કોષ (image) ના વડે તેને વધારી શકાય છે.View Solution
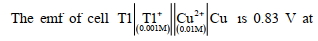
- 5પિગલીત કેલ્શિયમ હાઈડ્રાઈડ $(CaH_2)$ ના વિદ્યુત વિભાજન દરમિયાન હાઈડ્રોજન ક્યાં ઉત્પન્ન થાય?View Solution
- 6નીચે આપેલામાંથી ખોટા વિધાનોની સંખ્યા $........$ છે.View Solution
$A$. વિદ્યૃતકાર્ય કે જે પ્રક્રિયા અચળ દબાણ અને તાપમાન પર કરી શકે છે.તે પ્રક્રિયા ગિબ્સ ઊર્જા જેટલી છે.
$B$. $E ^{\circ}$ કોષ એ દબાણ ઉપર આધારિત છે.
$C$. $\frac{d E^\theta \text { cell }}{ dT }=\frac{\Delta_{ r } S ^\theta}{ nF }$
$D$. પોટેન્શિયલ તફાવતના વિરોધી સ્રોત દ્વારા જો કોષ પોટેન્શિયલ બરાબર સંતુલિત હોય તો કોષ ઊલટાવી શકાય તેવું કાર્ય કરે છે.
- 7ચાર ધાતુઓ $A, B, C$ અને $D$ ના પ્રમાણિત વિધુતધ્રુવ પોટેન્શિયલ $\left( {E_{{M^ + }/M}^\circ} \right)$ અનુક્રમે $-1.2\, V, 0.6\, V, 0.85\, V$ અને $- 0.76\, V$ છે. તો પોટેન્શિયલ લાગુ પાડતા ધાતુઓનો જમા થવાની ક્રમ જણાવો.View Solution
- 8આપેલ અર્ધકોષ પ્રક્રિયા માટે $pH = 5$ પર વિધુતધ્રુવ પોટેન્શિયલ શું થશે ?View Solution
$2 \mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{O}_{2}+4 \mathrm{H}^{\oplus}+4 \mathrm{e}^{-} ; \mathrm{E}_{\mathrm{red}}^{0}=1.23 \mathrm{V}$ અને $ - 5 \times {10^{ - 4}}\,V\,{K^{ - 1}}$ છે. કોષપ્રક્રિયા $(\mathrm{R}=8.314 \;\mathrm{J} \mathrm{mol}^{-1} \mathrm{K}^{-1} ; \text { Temperature }=298 \;\mathrm{K} ;$ ઓક્સિજન એ પ્રમાણિત વાતાવરણ દબાણ $1$ બાર હેઠળ છે)
- 9ઝિંક સલ્ફેટના દ્રાવણમાં $40$ મિનિટ માટે $5$ એમ્પિ. પ્રવાહ પસાર કરવામાં આવે તો કેથોડ પર જમા થતું ઝીંક નું મૂલ્ય કેટલું થાય?View Solution
- 10પાણીનાં વિધુતવિભાજન દરમિયાન $2.24\, dm^3$ જેટલો $O_2$ છૂટો પડે છે.તો આ જ પરિસ્થિતીમાં કેટલા .............. $\mathrm{dm}^{3}$ હાઇડ્રોજન છૂટો પડશે?View Solution
