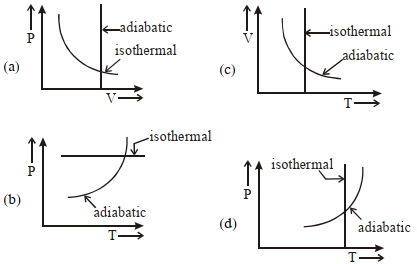
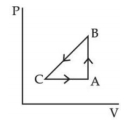
બે જુદી જુદી થર્મોડાયનેમિક પ્રક્રિયા માટે કયા ગ્રાફ સાચા છે?

JEE MAIN 2021, Medium
b
Option \((a)\) is wrong ; since in adiabatic process \(V \neq\) constant
Option \((a)\) is wrong ; since in adiabatic process \(V \neq\) constant
Option \((b)\) is wrong, since in isothermal process
\(T = constant\)
Option \((c)\) and \((d)\) matches isothermes and adiabatic formula :
\(TV ^{\gamma-1}=\) constant and \(\frac{ T ^{\gamma}}{ p ^{\gamma-1}}= constant\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$1$ મોલ આદર્શ વાયુ માટે, ઉષ્માગતિશાસ્ત્ર પ્રક્રિયાને $P-V$ આકૃતિ દ્વારા દર્શાવવામાં આવેલ છે. જે $V _{2}=2 V _{1}$ હોય તો તાપમાનનો ગુણોત્તર $T _{2} / T _{1}$ ........ છે.View Solution
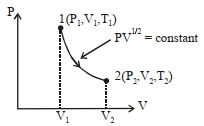
- 2ઉપરોકત $P-V$ આલેખ એક પરમાણ્વિક આદર્શ વાયુ ધરાવતાં થરમોડાઇનેમિક એન્જિન માટેની ચક્રીય પ્રક્રીયા દર્શાવે છે.એક ચક્રીય પ્રક્રીયા દરમિયાન ઉષ્મા-પ્રાપ્તિસ્થાનમાંથી મેળવેલ ઊર્જા _______ થશે.View Solution
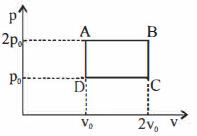
- 3$PV$ ડાયાગ્રામમાં દર્શાવ્યા મુજબ $27^{\circ} {C}$ તાપમાને રહેલ એક મોલ આદર્શ વાયુ ને ${A}$ થી ${B}$ લઈ જવામાં આવે છે. તંત્ર દ્વારા થતું કાર્ય $......\times 10^{-1} \,{J}$ જૂલ હશે.View Solution
[આપેલ : $R=8.3\, {J} /\,mole\,{K}, \ln 2=0.6931$ ] (નજીકના પૂર્ણાંકમાં)
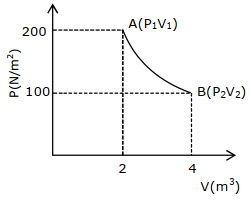
- 4એક આદર્શ વાયુના નમૂના પર આકૃતિમાં દર્શાવ્યા અનુસાર $ABCA$ ચક્રિય પ્રક્રિયા કરાવવામાં આવે છે. તે $AB$ ભાગ દરમ્યાન $40 \,J$ ઉષ્માનું શોષણ કરે છે, $BC$ ભાગ દરમ્યાન ઉષ્માનું શોષણ કરતી નથી, અને $CA$ ભાગ દરમ્યાન $60 \,J$ ઉષ્મા પાછી ફેંકે છે. જો $BC$ ભાગ દરમ્યાન વાયુ પર $50 \,J$ કાર્ય થાય છે. વાયુની $A$ સ્થાન આગળ આતંરિક ઊર્જા $1560 \,J$ છે. $CA$ ભાગ દરમ્યાન વાયુ દ્વારા થતું કાર્ય....... $J$ થશે.View Solution
- 5$300\; \mathrm{K}$ શરૂઆતના તાપમાને રહેલ એક મોલ દ્વિ પરમાણ્વિક વાયુ $(\gamma=1.4)$ ને પ્રથમ સમોષ્મી સંકોચન કરી તેનું કદ $\mathrm{V}_{1}$ થી $\mathrm{V}_{2}=\frac{\mathrm{V}_{1}}{16}$ થાય છે. પછી તેનું સમદાબી વિસ્તરણ કરતાં કદ $2 \mathrm{V}_{2} $ થાય છે. જો બધી જ પ્રક્રિયા ક્વાસી-સ્ટેટિક પ્રક્રિયા હોય તો વાયુનું અંતિમ તાપમાન($K$ માં) લગભગ કેટલું થાય?View Solution
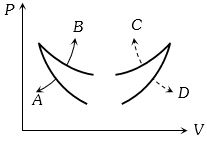
- 6View Solutionવાયુ માટે કયો આલેખ સમોષ્મી અને સમતાપીનો હશે.
- 7View Solutionનીચે આપેલા કયા તાપમાન માટે કાર્નોટ એન્જિનની કાર્યક્ષમતા મહત્તમ હોય.
- 8$STP$ એક લિટર હવાનું સમોષ્મી વિસ્તરણ થઈ તેનું કદ $3$ લિટર થાય છે.જો $\gamma=1.40,$ હોય તો હવા દ્વારા કેટલું કાર્ય થયું હશે?View Solution
$(3^{1.4}=4.6555)$ [હવાને આદર્શ વાયુ લો]
- 9View Solutionસમોષ્મી પ્રક્રિયામાં શું અચળ હોય?
- 10કાર્નોટ એન્જિનની કાર્યક્ષમતા $50\%$ અને ઠારણ વ્યવસ્થાનું તાપમાન $500\;K$ છે. જો ઉષ્મા પ્રાપ્તિસ્થાનનું તાપમાન અચળ રાખવામા આવે અને કાર્યક્ષમતા વધારીને $60\%$ કરવામાં આવે, તો ઠારણ વ્યવસ્થાનું જરૂરી તાપમાન ($K$ માં) કેટલું હશે?View Solution
