Enzyme -catalysed reactions follow first order kinectics.
therefore, Fall of concentration from \(1.28 \;\mathrm{mg}\, \mathrm{L}^{-1}\) to \(0.04\; \mathrm{mg} \,\mathrm{L}^{-1}\) involves five half-lives.
\(1.28 \stackrel{t_{1 / 2}}{\rightarrow} 0.64 \stackrel{t_{1 / 2}}{\rightarrow} 0.320 .16 \stackrel{t_{1 / 2}}{\rightarrow} 0.08 \stackrel{t_{1 / 2}}{\rightarrow} 0.04\)
therefore, Time required \(=5 \times t_{1 / 2}\)
\(=5 \times 138\; \mathrm{s}\)
\(=690\; \mathrm{s}\)
Download our appand get started for free
Similar Questions
- 1એક ઉષ્માક્ષેપક પ્રક્રિયા $X \rightarrow Y ,30 ,kJ\, mol ^{-1}$ સક્રિયકરણ શક્તિ ધરાવે છે. પ્રક્રિયા દરમ્યાન જે (શક્તિ) ઊર્જાનો ફેરફાર $\Delta E -20\, kJ$ હોય તો, પ્રતિવર્તી પ્રક્રિયા માટે સક્રિયકરણ શક્તિ $kJ$ માં ........... છે. (પૂર્ણાક જવાબ)View Solution
- 2$A \rightarrow 2 B + C$ પ્રક્રિયા માટે જ્યારે પ્રક્રિયક $A$ ની સાંદ્રતા અનુક્રમે $0.5$ અને $1.0\, mol \,L ^{-1}$ હોય ત્યારે તેમના અર્ધઆયુુષ્યો અનુક્રમે $100\, s$ અને $50\, s$ થાય છે. પ્રક્રિયાનો ક્રમ $....$ છે.View Solution
- 3પ્રથમ ક્રમ પ્રક્રિયાનો અદ્ય આયુ સમય $69.3$ સેકન્ડ છે તો જ્યારે પ્રક્રિયકની સાંદ્રતા $0.10 $ મોલ લીટર $^{-1} $ હોય તો પ્રક્રિયક અચળાંક દર કેટલો થશે?View Solution
- 4ચોક્કસ ઉત્સેચક-ઉત્પ્રેરિત પ્રક્રિયામાં પદાર્થનું અર્ધ આયુષ્ય સમય $138\, s$ છે , પદાર્થની સાંદ્રતા માટે જરૂરી સમય $1.28\, mg \,L^{-1}$ થી $0.04\, mg\, L^{-1}$ ....... $\sec$ શું થશેView Solution
- 5ઓર્ડર ${n}$ની પ્રક્રિયા માટે, વેગ અચળાંકનો એકમ શું છે?View Solution
- 6$N_2O_5\rightarrow 2NO_2 + \frac{1}{2} O_2 $ આપેલ પ્રક્રિયા માટેView Solution
$-\frac{d[{{N}_{2}}{{O}_{5}}]}{dt}={{K}_{1}}[{{N}_{2}}{{O}_{5}}]$ ,
$\frac{d[N{{O}_{2}}]}{dt}={{k}_{2}}[{{N}_{2}}{{O}_{5}}]$ ,
$\frac{d[{{O}_{2}}]}{dt}={{K}_{3}}[{{N}_{2}}{{O}_{5}}]$
તો $K_1$, $K_2$ અને $K_3 $ વચ્ચેનો સંબંધ શું થાય?
- 7પ્રક્રિયા, $a A +b B \rightarrow c C +d D$ માટે, આલેખ $\log \,k$ વિરૂધ્ધ $\frac{1}{ T }$ ને નીચે આપેલ છેView Solution
કયા તાપમાને $(K$ માં) પ્રક્રિયાનો વેગ અચળાંક $10^{-4} s ^{-1}$ થશે તે શોધો ?(નજીકના પૂર્ણાંકમાં રાઉન્ડ ઑફ)
[આપેલ : $500\, K$ પર, પ્રક્રિયાનો વેગ અચળાંક $10^{-5} s^{-1}$ છે.]
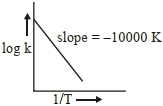
- 8જ્યારે તાપમાનનો ફેરફાર $293 K$ થી $313 K$ થાય તો ચોક્કસ પ્રક્રિયાનો દર ચતુષ્ક થાય છે. તો પ્રક્રિયાની સક્રિયકરણ ઊર્જા ......... $kJ\, mol^{-1}$ શોધો. $(R = 8.314 JK^{-1} mol^{-1}$)View Solution
- 9પ્રથમ ક્રમની પ્રક્રિયામાં પ્રક્રિયકની સાંદ્રતા $20$ મિનિટમાં $1.0\,M$ થી $0.25 \,M$ સુધી ઘટે છે. તો પ્રક્રિયાનો દર અચળાંક શું થશે?View Solution
- 10પ્રક્રિયાને ધ્યાનમાં લેતા, ${N_2}(g)\,\, + \,\,3{H_2}(g)\,\, \to \,2N{H_3}(g)\,\, $ તો $ \,\,\,\frac{{d[N{H_3}]}}{{dt}}\,\, $અને$ \, - \frac{{d[{H_2}]}}{{dt}}$વચ્ચેનો સમાનતાનો સંબંધ કયો છે?View Solution
