એક ઇલેકિટ્રક કીટલી ને $220\ V$ લગાડતાં તેમાંથી $4\ A$ પ્રવાહ વહે છે.આ કીટલીમાં ભરેલ $1\ kg $ પાણીને $20\,^o C$ થી ઊકળતા કેટલા ................ $min$ સમય લાગશે? પાણીનું ઉત્કલનબિદુ $100^o C$ છે.
AIPMT 2008, Medium
c
\(\text { Power }=220\, \mathrm{V} \times 4\, \mathrm{A}=880\, \text { watts. }=880\, \mathrm{J} / \mathrm{s}\)
\(\text { Power }=220\, \mathrm{V} \times 4\, \mathrm{A}=880\, \text { watts. }=880\, \mathrm{J} / \mathrm{s}\)
Heat needed to raise the temperature of \(1\,kg\) water through \(80\,^{o} \mathrm{C}\)
\( = ms.\,\Delta T \times 4.2\,{\rm{J}}/{\rm{cal}}\)
\( = 1000\,{\rm{g}} \times 1\,{\rm{cal}}/{\rm{g}} \times 80 \times 4.2\,{\rm{J}}/{\rm{cal}}\)
\( \therefore \) Time taken \({=\frac{1000 \times 1 \times 80 \times 4.2}{880}=\frac{336 \times 10^{3}}{880}} \)
\({=382\, \mathrm{s}=6.3\, \mathrm{min}}\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1જુદા જુદા $e.m.f.$ અને આંતરિક અવરોધ ધરાવતી બે બેટરીઓને એકબીજા સાથે શ્રોણીમાં અને બાહ્ય અવરોધ સાથે ધ્રુવને જોડવામાં આવે છે તો વિદ્યુતપ્રવાહ $1.0\,A$ થઈ જાય છે. બંને બેટરીઓના $e.m.f.$ નો ગુણોતર કેટલો છે.View Solution
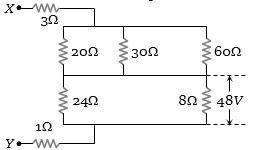
- 2આપેલ પરિપથમાં $X$ અને $Y$ વચ્ચે વોલ્ટેજ કેટલા ................ $volt$ થાય?View Solution
- 3View Solutionસુવાહકની વાહકતા.......છે.
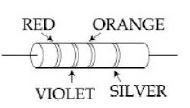
- 4View Solutionઆકૃતિમાં એક અવરોધ બતાવેલ છે. તેનું મૂલ્ય અને ટોલરન્સ અનુક્રમે __________ છે
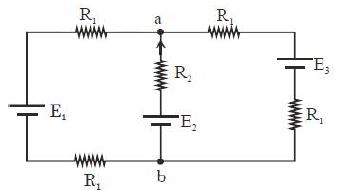
- 5આપેલ પરિપથમાં ${R_1} = 1.0\,\Omega $, ${R_2} = 2.0\,\Omega $, ${E_1} = 2\,V$ અને ${E_2} = {E_3} = 4\,V$ હોય તો બિંદુ $‘a’$ અને $‘b’$ વચ્ચે વિદ્યુતસ્થિતિમાનનો તફાવત કેટલો મળે?View Solution
- 6કોપરની પટ્ટી અને જર્મેનિયમની પટ્ટીને રૂમ તાપમાનથી $80\, K$ તાપમાન સુધી ઠંડી પાડવામાં આવે તો તેમના અવરોધ વિષે શું કહી શકાય?View Solution
- 7$4\,\Omega $ અવરોધના ત્રણ તારમાથી સમબાજુ ત્રિકોણ બનાવવામાં આવે છે. બે ખૂણા વચ્ચેનો સમતુલ્ય અવરોધ કેટલો થાય?View Solution
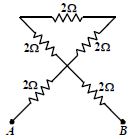
- 8આકૃતિમાં દર્શાવેલ પરિપથમાં $A$ અને $B$ વચ્ચેનો વચ્ચેનો સમતુલ્ય અવરોધ ($\Omega$ માં) કેટલો થાય?View Solution
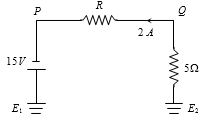
- 9આપેલ પરિપથમા $P$ અને $Q$ વચ્ચે વોલ્ટેજ કેટલા ................... $V$ થાય?View Solution
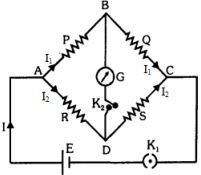
- 10View Solutionવ્હીસ્ટનબ્રીજના કિસ્સામાં સંતુલન સ્થિતિ…..