Rate of formation of \(B\) is
\(\frac{\mathrm{d}[\mathrm{B}]}{\mathrm{dt}}=\mathrm{k}_1[\mathrm{~A}]-\mathrm{k}_2[\mathrm{~B}]\)
\(0=\mathrm{k}_1[\mathrm{~A}]-\mathrm{k}_2[\mathrm{~B}]\)
\(\left(\frac{\mathrm{k}_1}{\mathrm{k}_2}\right)[\mathrm{A}]=[\mathrm{B}]\)
Download our appand get started for free
Similar Questions
- 1શ્વાસ વિશ્લેષકમાં થતી પ્રક્રિયા, વ્યક્તિના રક્ત પ્રવાહમાં આલ્કોહોલનું સ્તર નક્કી કરવા માટે વપરાતું ઉપકરણ છેView Solution
$2 {~K}_{2} {Cr}_{2} {O}_{7}+8 {H}_{2} {SO}_{4}+3 {C}_{2} {H}_{6} {O} \rightarrow 2 {Cr}_{2}\left({SO}_{4}\right)_{3}+$
$3 {C}_{2} {H}_{4} {O}_{2}+2 {~K}_{2} {SO}_{4}+11 {H}_{2} {O}$
જો ${Cr}_{2}\left({SO}_{4}\right)_{3}$નો દેખાવનો દર $2.67 \,{~mol}$ $\min ^{-1}$ ચોક્કસ સમયે, ${C}_{2} {H}_{6} {O}$નો એક જ સમયે ગાયબ થવાનો દર $....$ ${mol}\, {min}^{-1}$ છે. (નજીકના પૂર્ણાંકમાં)
- 2જો તાપમાન $300\, K$ થી $310\, K$ કરવામાં આવે તો રાસાયણિક પ્રક્રિયાનો વેગ બમણો થાય છે. આ પ્રક્રિયાની સક્રિયકરણ શક્તિ .......... $kJ\, mol^{-1}$ થશે .View Solution
$(R= 8.314\,JK^{-1} \,mol^{-1}$ and $\log 2=0.301)$
- 3અર્ધ આયુષ્યના કિરણોત્સર્ગી નમૂના માટે ત્વરિત સમયે કિરણોત્સર્ગી વિઘટનનો દર$2.2 \times 10^{9}\; \mathrm{s}$$10^{10} \;\mathrm{s}^{-1}$છે .તે ઇન્સ્ટન્ટમાં તે નમૂનામાં કિરણોત્સર્ગી અણુઓની સંખ્યા કેટલી છે ?View Solution
- 4$t_{1/2}$ અને $ n^{th}$ ક્રમની પ્રક્રિયા માટે સાંદ્રતા વિરૂદ્ધ સમય નો આલેખ સીધી રેખામાં છે. જ્યારે સાંદ્રતા $2$ મોલ $L^{-1}$ હોય ત્યારે આ પ્રક્રિયાને $50\%$ પૂર્ણ થવા $10$ મિનિટ લાગે છે. આ પ્રક્રિયા $4$ મોલ $L^{-1}$ એ $t$ સમયમાં $50\%$ વિઘટન થાય તો $n$ અને $t$ અનુક્રમે.....View Solution
- 5જો દર અચળાંક $1.155 \times 10^{-3}$ સેકન્ડ $^{-1}$ હોય તો કેટલી સેકન્ડ પછી પ્રક્રિયકની સાંદ્રતા પ્રથમ ક્રમની પ્રક્રિયામાં અડધી થશે?View Solution
- 6એક પ્રક્રિયા વેગ-અચળાંક $K_1$ અને $K_2$ અનુક્રમે $10^{16}.e^{-2000/T}$ અને $10^{15}.e^{-1000/T } $ છે, તો ક્યા તાપમાને $K_1 = K_2$ થશે ?View Solution
- 7પ્રથમ ક્રમ પ્રક્રિયાનો વેગ પ્રક્રિયાની શરૂઆત થયા પછી $10$ minutes પર $0.04 \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~S}^{-1}$ છે અને $20 \ minutes$ પર $0.03 \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}$ છે. પ્રક્રિયાનો અર્ધ આયુષ્ય _______________ $minutes$ છે. (આપેલ : $\log 2=0.3010, \log 3=0.4771)$View Solution
- 8View Solutionપ્રક્રિયાની સક્રિયકરણ ઊર્જા......
- 9આપેલ પ્રક્રિયા માટે નીચે આપેલ માહિતીને ધ્યાનમાં લો.View Solution
$2 \mathrm{HI}_{(\mathrm{g})} \rightarrow \mathrm{H}_{2(\mathrm{~g})}+\mathrm{I}_{2(\mathrm{~g})}$
પ્રક્રિયાનો ક્રમ................ છે.
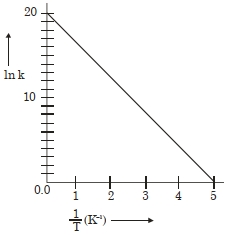
$1$ $2$ $3$ $\mathrm{HI}\left(\mathrm{mol} \mathrm{L}^{-1}\right)$ $0.005$ $0.01$ $0.02$ Rate $\left(\mathrm{mol} \mathrm{L}^{-1} \mathrm{~s}-1\right)$ $7.5 \times 10^{-4}$ $3.0 \times 10^{-3}$ $1.2 \times 10^{-2}$ - 10એક પ્રક્રિયા માટે, $\ln K$ વિરુદ્ધ $\frac{1}{ T }$ નો આલેખ નીચે આપેલો છે. પ્રક્રિયાની સક્રિયકરણ ઊર્જા $......cal$ $mol ^{-1}$ (નજીકના પૂર્ણાંક)View Solution
(આપેલું છે: $R =2\,cal\,K ^{-1}\,mol ^{-1}$ )