એક પ્રથમક્રમ પ્રક્રિયા $A \rightarrow$ નીપજો માટે, $A$ની પ્રારંભિક સાંદ્રતા $0.1\,M$ છે, જે $5$ મિનિટો પછી $0.001 \,M$ થાય છે. પ્રક્રિયા માટે વેગ અચળાંક $min ^{-1}$માં શોધો.
NEET 2022, Medium
a
\(A \rightarrow\) Products
\(A \rightarrow\) Products
Initial conc. \(A_{0}=0.1\, M\)
Conc. After \(5 \min A_{t}=0.001\, M\) \(t =5\, min\).
For first order reaction
\(K =\frac{2.303}{t} \log \left(\frac{A_{0}}{A_{t}}\right)\)
\(=\frac{2.303}{5} \log \left(\frac{0.1}{0.001}\right)\)
\(K =0.9212 \,\min ^{-1}\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1મોટાભાગનાં કિસ્સામાં, $10\,K$ તાપમાનની માટે, અચળ વેગ દર સતત બમણા થાય છે. આ કોના કારણે છે ?View Solution
- 2$NO$ અને $Br_2$ વચ્ચેની પ્રક્રિયાથી $NOBr$ બનવાની પ્રક્રિયાની કાર્યપ્રણાલી નીચે મુજબ છે. :View Solution
$NO(g) + Br_2 (g) \rightleftharpoons NOBr_2 (g)$
$NOBr_2(g)+ NO(g)\longrightarrow 2NOBr(g)$
જો બીજો તબક્કો ધીમો તબક્કો હોય, તો $NO(g)$ ની સાપેક્ષે પ્રક્રિયા ક્રમ ........ થશે.
- 3જો પ્રક્રિયક $'A'$ની સાંદ્રતા બમણી કરવાથી પ્રક્રિયા વેગ $4$ ગણો વધે છે અને $'A'$ ની સાંદ્રતા ત્રણ ગણી વધારતા $9$ ગણો વધે છે, તો દર કોના પ્રમાણમાં છે?View Solution
- 4પ્રક્રિયા $\frac{1}{2} A \rightarrow 2B$ માટે $A$ ના દૂર થવાનો દર એ $B$ ના ઉત્પન્ન થવાના દર સાથે ......... સંબંધ ધરાવે છે.View Solution
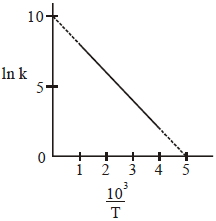
- 5પ્રક્રિયાનો વેગ અચળાંક $(k)$ જુદા-જુદા તાપમાન $(T)$ પર માપવામાં આવે છે અને આપેલ આકૃતિમાં માહિતી આલેખ દ્વારા આપવામાં આવેલ છે.પ્રક્રિયાની સક્રિયકરણ ઊર્જા $kJ\, mol ^{-1}$માં થશે?View Solution
($R$ એ વાયુ અચળાંક છે)
- 6$Pt$ની સપાટી પર $NH _{3}$નું વિઘટન શૂન્ય ક્રમની પ્રક્રિયા છે. જો વેગ અચળાંકનું મૂલ્ય $2 \times 10^{-4}\,mole $ $liter^{-1}\, sec ^{-1}$ છે. $N _{2}$ અને $H _{2}$ના વેગ અનુક્રમે છે?View Solution
- 7પ્રકાશરાસાયણિક પ્રક્રિયા $AB + hv \to AB^*$માં $AB$ માટે $'I'$ શોષિત પ્રકાશની તીવ્રતા છે અને $C$ સાંદ્રતા છે,તો $AB^*$ની રચનાનો દર સીધો કોના પ્રમાણમાં છે?View Solution
- 8$[ A ]$ પ્રક્રિયક $\rightarrow$ $[ B ]$ નીપજView Solution
જો સંયોજન $[B]$નું બનવું એ પ્રથમક્રમ ગતિકીને અનુસરતું હોય તો, અને $70 \,mins$ પછી $[A]$ ની સાંદ્રતા તેની પ્રારંભિક સાંદ્રતા કરતા અડધી મળી આવેલ છે. પ્રક્રિયાનો વેગ અચળાંક એ $x \times 10^{-6}\, s ^{-1}$ છે. તો $x$ નું મૂલ્ય નજીકના પૂર્ણાંકમાં $.....$ છે.
- 9જ્યારે તાપમાન વધીને $298\,K$ થી $308 \,K$ થાય ત્યારે તેનો દર બમણો થાય તો પ્રક્રિયાની સક્રિયકરણ ઊર્જા ........... $kJ\, mol^{-1}$ થાય.View Solution
- 10View Solutionઓરડાના તાપમાને હાઇડ્રોજન અને ઓક્સિજનનુ મિશ્રણ ખૂબ સ્થાયી છે. પરંતુ સ્પાર્ક કરવાથી તરત જ વિસ્ફોટ થાય છે. કારણ કે .........
