$CH_3COCH_{3(aq)} + Br_{2(aq)} \rightarrow $$CH_3COCH_2Br_{(aq)} + H^+_{(aq)}+ Br^-_{(aq)}$
નીચેની પ્રક્રિયા સાંદ્રતા પરથી આ ગતિકીય માહિતી મળે છે.
શરૂઆતની સાંદ્રતા, $M$
| $[CH_3COCH_3]$ | $[Br_2]$ | $[H^+]$ |
| $0.30$ | $0.05$ | $0.05$ |
| $0.30$ | $0.10$ | $0.05$ |
| $0.30$ | $0.10$ | $0.10$ |
| $0.40$ | $0.05$ | $0.20$ |
$Br_2$ ના દૂર થવાનો શરૂઆતનો દર $Ms^{-1}$ માં નીચે મુજબ છે.
$5.7 \times 10^{-5} ,$ $5.7 \times 10^{-5} ,$ $1.2 \times 10^{-5} ,$ $3.1 \times 10^{-5}$
આ માહિતીને આધારે વેગ સમીકરણ ...... થશે.
By comparing the rate and concentration, the order of the reaction can be calculated. Let the rate of the reaction wrt \(\left[\mathrm{CH}_{3} \mathrm{COCH}_{3}\right],\left[\mathrm{Br}_{2}\right]\) and \(\left[\mathrm{H}^{+}\right]\) are \(\mathrm{x}, \mathrm{y}\) and \(z\) respectively.
Thus,
Rate \(\propto\left[\mathrm{CH}_{3} \mathrm{COOH}_{3}\right]^{\mathrm{X}}\left[\mathrm{Br}_{2}\right]^{\mathrm{Y}}\left[\mathrm{H}^{+}\right]^{z}\)
\(5.7 \times 10^{-5}=[0.30]^{x}[0.05]^{y}[0.05]^{z}\) ..... \((i)\)
\(5.7 \times 10^{-5}=[0.30]^{\times}(0.10)^{y}(0.05)^{z}\) ..... \((ii)\)
\(1.2 \times 10^{4}=[0.30)^{x}(0.10)^{y}(0.10)^{z}\) ..... \((iii)\)
\(3.1 \times 10^{-4}=[0.40]^{x}(0.05)^{y}(0.20)^{z} \) ..... \((iv)\)
From eqs \((i)\) and \((ii)\)
\(\mathrm{v}=0\)
From eqs \((ii)\) and \((iii)\)
\(z=1\)
From eqs \((i)\) and \((iv)\)
\(x=1\)
Thus, rate law \(\propto\left[\mathrm{CH}_{3} \mathrm{COCH}_{3}\right]\left[\mathrm{H}^{+}\right]\)
\(=\mathrm{k}\left[\mathrm{CH}_{3} \mathrm{COCH}_{3}\right]\left[\mathrm{H}^{+}\right]\)
Download our appand get started for free
Similar Questions
- 1${A} \rightarrow {B}$ પ્રક્રિયા માટે, દર અચળાંક ${k}$ એ ${s}^{-1}$માં $\log _{10} {k}=20.35-\frac{\left(2.47 \times 10^{3}\right)}{{T}}$ દ્વારા આપવામાં આવે છે. સક્રિયકરણની ઊર્જા $.....$ ${kJ} \,{mol}^{-1}$ છે. (નજીકના પૂર્ણાંકમાં) $\left[\right.$ આપેલ છે $\left.: {R}=8.314\, {~J}\, {~K}^{-1}\, {~mol}^{-1}\right]$View Solution
- 2View Solutionપ્રક્રિયા દરની સંઘાત પદ્ધતિના આધારે.....
- 3આર્હેંનિયસ સમીકરણમાં પ્રક્રિયાનાં દરને $k\, = \,\,A{e^{ - {E_a}/RT}}$તરીકે સમજાવાય. જ્યાં $E_a$ શું દર્શાવે છે?View Solution
- 4પ્રથમ ઓર્ડર પ્રક્રિયા માટે $A \rightarrow 2 B$માં $100$ મિનિટ પછી $1$ મોલ પ્રક્રિયક $A$ પછી $B$ ના $0.2$ મોલ્સ આપે છે. પ્રક્રિયાનું અર્ધઆયુષ્ય $.....\,min$ છે. [ઉપયોગ કરો $: \ln 2=0.69, \ln 10=2.3$View Solution
$log$નો ગુણધર્મ $\ln \left(\frac{{x}}{{y}}\right)=\ln {x}-\ln {y}$
- 5નીચેના આંકડા મુજબ, પ્રક્રિયાના એન્થાલ્પી ફેરફારની તીવ્રતાView Solution
${A}+{B} \rightarrow {M}+{N}$ $......$ ${kJ} {mol}^{-1}$ બરાબર છે. (નજીકના પૂર્ણાંકમાં)
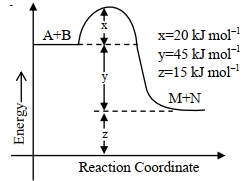
- 6$_{92}U^{235}$ નો ક્ષય.......... ક્રમની પ્રક્રિયા છે.View Solution
- 7એક પ્રક્રિયા $2A+ B \rightarrow$ નીપજ, ની ગતિકી અભ્યાસ દરમ્યાન નીચેના પરિણામો મળ્યા :View Solution
પ્રયોગ $[A]$
($mol\, L^{-1})$
$[B]$
($mol\, L^{-1})$
પ્રક્રિયાની શરૂઆતનો દર
$(mol\, L^{-1}$ $min^{-1})$
$I$ $0.10$ $0.20$ $6.93 \times {10^{ - 3}}$ $II$ $0.10$ $0.25$ $6.93 \times {10^{ - 3}}$ $III$ $0.20$ $0.30$ $1.386 \times {10^{ - 2}}$ $A$ અડધો વપરાય તે માટેનો સમય મિનિટમાં કેટલો થાય
- 8પ્રક્રિયા $2N_2O_5\,(g) \to 4NO_2\,(g) + O_2\,(g)$ એ પ્રથમ ક્રમની ગતિકીને અનુસરે છે. ફ્ક્ત $N_2O_5$ ધરાવતા પાત્રના દબાણમાં $30$ $min$ એ $50$ $mm$ $Hg$ થી વધીને $87.5$ $mm$ $Hg$ થાય છે. તો $60$ $min$ બાદ વાયુઓ દ્વારા દર્શાવાતુ દબાણ કેટલુ થશે ? (તાપમાન અચળ રહે છે તેમ ધારો)View Solution
- 9$H_2O_2$ ના વિઘટનની પ્રક્રિયાનો વેગ અચળાંક $3.66 \times 10^{-3}\,s^{-1}$ છે. જો $H_2O_2$ ની શરૂઆતની સાંદ્રતા $0.882\, M$ હોય, તો ........ $\sec$ એ તેની સાંદ્રતા $0.600\,M$ થશે.View Solution
- 10$87.5 \%$ પ્રક્રિયા પૂર્ણ થવા માટે જરૂરી સમય $t_{87.5}$ છે અને $50 \%$ પ્રક્રિયા પૂર્ણ થવા માટે જરૂરી સમય $t _{50}$ છે. $t _{87.5}=x \times t _{50}$ સંબંધ ધરાવે છે.તો $x$ નું મૂલ્ય $........$ છે.View Solution
