\(\mathrm{H}_{2}+\mathrm{Cl}_{2} \rightarrow 2 \mathrm{HCl}\)
\(\Delta H_{\text {reaction }}=\Sigma(\mathrm{B.E})_{\text {reactant }}-\Sigma(\mathrm{B.E})_{\text {product }}\)
\(=\left[(\mathrm{B.E})_{\mathrm{H}-\mathrm{H}}+(\mathrm{B.E})_{\mathrm{Cl}-\mathrm{Cl}}\right]-\left[2 \mathrm{B.E}_{(\mathrm{H}-\mathrm{Cl})}\right]\)
\(=434+242-(431) \times 2\)
\(\Delta H_{\text {reaction }}=-186 \mathrm{kJ}\)
Heat of formation is the amount of heat absorbed or evolved when one mole of substance is directly obtained from its constituent element.
Hence, enthalpy of formation of \(\mathrm{HCl}=-186 / 2\; \mathrm{kJ}\)
\(=-93 \;\mathrm{kJ} \mathrm{mol}^{-1}\)
Download our appand get started for free
Similar Questions
- 1View Solution...... વચ્ચેની પ્રક્રિયામાં તટસ્થીકરણ એન્થાલ્પીનું મૂલ્ય સૌથી વધુ હોય છે.
- 2સૂચી $-I$ ને સૂચી $-II$ સાથે જોડો.View Solution
સૂચી$-I$ સૂચી$-II$ $(A)$ સ્વયંસ્ફુરિત પ્રક્રમ $(I)\,\Delta H < 0$ $(B)\,\Delta P =0\;\Delta T =0$ સાથે પ્રક્રમ $(II)\,\Delta G _{ T , P } < 0$ $(C)\,\Delta H _{reaction}$ $(III)$ સમતાપિય અને સમદાબીય પ્રક્રિયા $(D)$ ઉષ્માક્ષેપક પ્રક્રિયા $(IV)$ [પક્રિયક અણુની બંધ ઉર્જાઓ] - [નીપજ અણુંની બંધ ઉર્જાઓ] નીચે આપેલ વિકલ્પમાંથી યોગ્ય ઉત્તર પસંદ કરો.
- 3View Solutionનીચેના પૈકી ખોટી સરખામણી જણાવો.
- 4$300\,K$ પર સ્વતંત્ર પ્રક્રમો માટે, સ્વયંભૂ ન થતી (આપમેળે ના થાય તેવી) પ્રક્રમોની સંખ્યા નીચે આપેલામાંથી $.........$ છે.View Solution
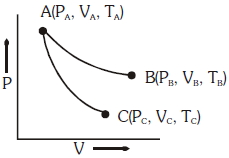
પ્રક્રમ $\Delta H / kJ\,mol ^{-1}$ $\Delta S / J K^{-1}$ $A$ $-25$ $-80$ $B$ $-22$ $40$ $C$ $25$ $-50$ $D$ $22$ $20$ - 5આકૃતિમાં બતાવ્યા પ્રમાણે સમતાપી અને સમોષ્મી પરિસ્થિતિઓ હેઠળ આદર્શ વાયુની પ્રતિવર્તી વિસ્તરણ બતાવવામાં આવેલ છે,View Solution
$AB \to$ સમતાપી વિસ્તરણ
$AC \to$ સમોષ્મી વિસ્તરણ
તો નીચેનામાંથી કયો વિક્લપ સાચો નથી?
- 6$H - H$ અને $Cl - Cl$ ની બંધ-ઊર્જા અનુક્રમે $430$ કિલો જૂલ મોલ$^{-1}$ અને $240$ કિલો જૂલ મોલ$^{-1}$ છે. $HCl$ ની $\Delta H_f$ (સર્જનઉષ્મા) $-90$ કિલો જૂલ મોલ$^{-1}$ હોય, તો $H - Cl $ બંધ-ઊર્જા કેટલા ....... કિલો જૂલ મોલ$^{-1}$ હશે ?View Solution
- 7મિસેલ બનાવટ માટે નીચે આપેલા વિધાનોમાંથી કયું સાચુ છે ?View Solution
$A.$ મિસેલ બનાવટ એ એક ઉષ્માક્ષેપક પ્રક્રમ છે.
$B.$ મિસેલ બનાવટ એ એક ઉષ્માશોષક પ્રક્રમ છે.
$C.$ એન્ટ્રોપી ફેરફાર ધન છે.
$D$ એન્ટ્રોપી ફેરફાર ઋણ છે.
- 8View Solutionનીચેનામાંથી કયું ઉષ્માગતિશાસ્ત્ર પ્રાણાલીનો અવસ્થા વિધેય નથી ?
- 9નીચેની પ્રક્રિયામાં $5.67$ મોલ $HCl$ વાયુના સર્જનમાં કેટલા ......$kJ$ એન્થાલ્પી ફેરફાર સંકળાયેલો છે ?${H_{2\left( g \right)}} + C{l_{2\left( g \right)}} \to 2HC{l_{\left( g \right)}}\,;\Delta H = - 184.6\,kJ$View Solution
- 10પ્રતિમોલ ઈથેનોલની બાષ્પાયન એન્થાલ્પી કેટલા ............ $\mathrm{kJ/mol}$ થશે ? $(b.p. = 79.5\,^oC$ અને $\Delta S$ $= 109.8 $ $JK^{-1}\, mol^{-1}$) છે.View Solution
