જો ચક્રીય પ્રક્રિયામાં, $Q, E$ અને $W$ અનુક્રમે ઉમેરેલી ઉષ્મા, આંતરિક ઊર્જામાં ફેરફાર અને કરવામાં આવેલ કાર્ય દર્શાવે છે, તો ....
AIPMT 2008, Easy
c
Internal energy depends only on the initial and final states of temperature and not on the path. In a cyclic process, as initial and final states are the same, change in internal energy is zero. Hence \(E\) is \(\Delta U\), the change in internal energy.
Internal energy depends only on the initial and final states of temperature and not on the path. In a cyclic process, as initial and final states are the same, change in internal energy is zero. Hence \(E\) is \(\Delta U\), the change in internal energy.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1એક રેફ્રિજરેટરનું અંદરનું તાપમાન $t_2\, ^o C$ છે અને ઓરડાનું તાપમાન $t_1 \,^o C$ છે. આદર્શ અવસ્થામાં પ્રતિજૂલ વિદ્યુતઊર્જાનો વ્યય થાય ત્યારે, ઓરડાને આપેલી ઉષ્માનું મૂલ્ય કેટલું હશે?View Solution
- 2View Solutionનીચેનામાંથી કઇ પ્રક્રિયામાં કાર્ય શૂન્ય થાય.
- 3View Solutionબે વાયુઓ તાપીય સંતુલનમાં હોવાનું કહેવાય છે જ્યારે તેઓ સમાન ધરાવે છે.
- 4View Solutionપ્રતિવર્તી એન્જિન ચક્રનો તાપમાન એન્ટ્રોપી આલેખ નીચે આપેલ છે તો તેની કાર્યક્ષમતા કેટલી થાય?
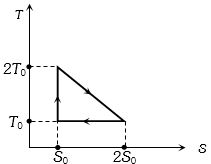
- 5વાયુ ($\gamma=\frac{5}{3}$ ધરાવતા) માટે સમતાપનો ઢાળ $3 \times 10^5 \,N /m ^2$ છે. જો એ જ વાયુ સમોષ્મી ફેરફારમાંથી પસાર થતો હોય તો તે ક્ષણે સમોષ્મી સ્થિતિસ્થાપકતા ........ $\times 10^5 N / m ^2$ છે ?View Solution
- 6View Solutionથર્મોડાઇનેમિકસનો પ્રથમ નિયમ શેના સંરક્ષણને સંબંધિત છે.
- 7તંત્રને $300$ કેલરી ઉષ્મા આપતા $600\,J$ કાર્ય થાય છે, તો તંત્રની આંતરિક ઊર્જામાં ફેરફાર $( J =4.18$ $Joules / cal )$ (જૂલ માં)View Solution
- 8$40\%$ કાર્યક્ષમતા ધરાવતા કાર્નોટ એન્જિન માટે ઠારણ-વ્યવસ્થાનું તાપમાન $300 K$ છે. તેની ઠારણ-વ્યવસ્થાનું તાપમાન અચળ રાખીને, કાર્યક્ષમતા મૂળ કાર્યક્ષમતા કરતાં $50\%$ વધારવા માટે પ્રાપ્તિસ્થાન તાપમાન ..... $K$ વધારવું પડે ?View Solution
- 9વાયુનો નમૂનો $V_1$ કદથી $V_2$ કદમાં વિસ્તરે છે. વાયુ દ્વારા થતું કાર્ય મહતમ હોય જ્યારે વિસ્તરણ .......... હોય.View Solution
- 10View Solutionકાર્નોટ ચક્રમાં ......... નો સમાવેશ થાય છે.
