જો કોઇ ચોક્કસ પ્રક્રિયા માટે $450\, K$ પર $\Delta_{ r } H$ એ $30\, kJ\, mol ^{-1}$ હોય તો આ જ પ્રકિયા આ જ તાપમાને સ્વયંભૂ બને તે માટે $\Delta_{ r } S$ નું મૂલ્ય $\left( J K^{-1} mol ^{-1}\right.$ માં) જણાવો.
NEET 2020, Medium
b
For spontaneous reaction, \(\Delta_{r} G\) must be less than zero.
For spontaneous reaction, \(\Delta_{r} G\) must be less than zero.
So, \(\Delta_{r} G=\Delta_{r} H-T \Delta_{r} S<0\)
or, \(\Delta_{r} S >\frac{\Delta_{ r } H }{ T }\)
\(>\frac{30,000}{450}=66.67 J\)
\(\therefore\) For reaction to be spontaneous, the value of \(\Delta_{r} S\) must be greater than 66.67 J
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1એક નિરાળી પ્રણાલીમાં આદર્શવાયુનું પ્રતિવર્તી અને અપ્રતિવર્તી એમ બંને રીતે વિસ્તરણ કરવામાં આવે છે. જો $T_i$ એ પ્રારંભિક તાપમાન અને $T_f$ એ અંતિમ તાપમાન હોય. તો નીચેનામાંથી ક્યુ વિધાન સાચુ છે?View Solution
- 2$1000\, K$ પર $CD_2O$ ની મોલર ઉષ્માક્ષમતા $(C_p)$ $10$ કેલરી છે. તો અચળ દબાણે $32\, g$ $CD_2O$ ને $1000\, K$ થી $100\, K$ તાપમાને ઠંડુ પાડવા સાથે સંકળાયેલ એન્ટ્રોપી ફેરફાર ....$cal\, deg^{-1}$. ($D\, =$ ડયુટેરિયમ, પરમાણ્વિય દળ $= 2\,u$ )View Solution
- 3$1$ મોલ આદર્શ વાયુના તાપમાનમાં $2\,^oC$ નો વધારો કરતા થતુ કાર્ય .........$J$ થશે.View Solution
- 4ડાઇમરાઇઝેશન પ્રક્રિયા માટે,View Solution
$2 A ( g ) \rightarrow A _{2}( g )$
$298\, K$ પર $\Delta U^ \ominus,=-20\, kJ\, mol ^{-1}, \Delta S \odot=-30\, J$$K ^{-1}\, mol ^{-1},$ પછી $\Delta G ^{\ominus}$ ........$J$ હશે?
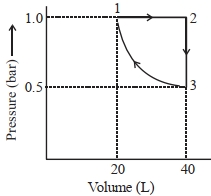
- 5એક મોલ આદર્શ એકપરમાણ્વીય વાયુને આલેખમાં દર્શાવ્યા પ્રમાણે ફેરફાર કરેલ છે. થયેલ કાર્યની માત્રા (પ્રણાલી વડે અથવા પ્રણાલી ઉપર) $..........J$ છે. (નજીકનો પૂર્ણાક) Given : $\log 2=0.3, \ln 10=2.3$View Solution
- 6View Solutionપ્રક્રિયાનો સંતુલન અચળાંક કોની સાથે સંબંધિત છે?
- 7$S + \frac{3}{2}{O_2}\, \to \,S{O_3} + 2x\,$ કિલોકેલેરી $\Delta H\,\, = \,\, - y$ કિલોકેલેરી, $S{O_2} + \frac{1}{2}{O_2} \to S{O_3} + y$ કિલોકેલેરી $\Delta H = -2x$ કિલોકેલરી, તો $SO_2$ ની સર્જન ઉષ્મા $= ......$View Solution
- 8$45.0\, g$ સિલિકોનના તાપમાનમાં $6\,^oC$ નો વધારો કરવા $192\,J$ ઉષ્માની જરૂર પડે તો તેની વિશિષ્ટ ઉષ્માક્ષમતા.....View Solution
- 9પ્રમાણિત રિડક્ષન પોટેન્શિયલ $M ^{+} / M$ કે જે રિડ્યુસીંગ સામર્થ્ય (શક્તિ)નું માપ આપે છે, ના ઉપર નિર્ભર નથી તે $........$View Solution
- 10શરીરનું તાપમાન $1 \,K$ વધારવા માટે જરૂરી ઉષ્માને કહેવામાં આવે છેView Solution
