નીચેના ઉષ્મારાસાયણિક સમીકરણોના આધારે
${H_2}O(g) + C(s) \to CO(g) + {H_2}(g);\,\Delta H = 131\,kJ$$CO(g) + \frac{1}{2}{O_2}(g) \to C{O_2}(g);\Delta H = - 282\,kJ$
${H_2}(g) + \frac{1}{2}{O_2}(g) \to {H_2}O(g);\,\Delta H = - 242\,kJ$
$C(s) + {O_2}(g) \to C{O_2}(g);\,\Delta H = X\,kJ$
$X$ નું મૂલ્ય ......$kJ$
AIPMT 1992, Medium
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$27\,^oC$ તાપમાને પ્રવાહી પાણીમાંથી બાષ્પ બને ત્યારે થતો એન્થાલ્પી ફેરફાર $30\, kJ/mol$ હોય તો આ પ્રક્રમ માટે એન્ટ્રોપી ફેરફારનું મૂલ્ય કેટલા ............ $\mathrm{J\,mol}^{-1}\, \mathrm{K}^{-1} $ હશે ?View Solution
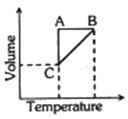
- 2કોઈ વાયુના પાંચ મોલને શ્રેણીમાં થતા ફેરફારના ઘટનાક્રમમાં મૂકવામાં આવેલ છે. જે નીચે આપેલા આલેખ વડે દર્શાવી શકાય છે, તો આ આલેખમાં $A$ $\rightarrow$ $B$, $B$ $\rightarrow$ $C$ અને $C$ $\rightarrow$ $A$ અનુક્રમે શું હશે ?View Solution
- 3પ્રક્રિયાની ઉષ્મા નીચે પ્રમાણે છે : તો પ્રક્રિયા $C_2H_2$ + $H_2$ $\rightarrow$ $C_2H_4$ માટે ઉષ્મા ફેરફાર......$ K\, cal$View Solution
$(i)\,\,\Delta H_f^o\,\,of\,{H_2}{O_{(\ell )}}\, = \,\, - 68.3\,K\,\,cal\,\,mo{l^{ - 1}}$
$(ii)\,\,\Delta H_{comb}^o\,\,of\,{C_2}{H_2}\, = \,\, - 337.2\,K\,\,cal\,\,mo{l^{ - 1}}$
$(iii)\,\,\Delta H_{comb}^o\,\,of\,\,{C_2}{H_4}\,\, = \,\, - \,363.7\,\,K\,\,cal\,\,mo{l^{ - 1}} $
- 4$15.5\, g$ પ્રોપેનના દહનથી કેટલા $.......kJ$ ઉષ્મા ઉત્પન્ન થાય ? ${C_3}{H_8} + 5{O_2} \to 3C{O_2} + 4{H_2}O\,;\,\Delta {H^o} = - 2219\,kJ/mol$View Solution
- 5અચળ દબાણે પાણીની મોલર ઊર્જા $75$ $JK^{-1}$ $mol^{-1}$ છે. જ્યારે $1.0 \,kJ$ ઊર્જા જે મુક્ત રીતે વિસ્તરી શકતા $100\, g$ પાણીને આપવામાં આવે તો પાણીના તાપમાનમાં થતો વધારો ............. $\mathrm{K}$View Solution
- 6વાયુમય પ્રક્રિયા માટે $A_{(g)} + 3B_{(g)} \rightarrow 3C_{(g)} + 3D_{(g)}$ $27\,^oC$ એ $\Delta U=17 \,Kcal$ છે. ધારો કે $R = 2 \,cal \,K$$^{-1}$ મોલ$^{-1}$ છે તો ઉપરની પ્રક્રિયા માટે $\Delta H$ નું મુલ્ય .......$Kcal$ થશે.View Solution
- 7View Solutionનીચેના પૈકી કયો વાયુ સૌથી વધારે દહન ઉષ્મા ધરાવે છે ?
- 8$X_2, Y_2$ તથા $XY_3$ ની પ્રમાણિત એન્ટ્રોપી અનુક્રમે $60, 40$ અને $50\, J\,K^{-1}\,mol^{-1}$ છે. પ્રક્રિયા $\frac{1}{2}{X_2} + \frac{3}{2}{Y_2} \to X{Y_3}$ માટે $\Delta H = - 30\,kJ$ હોય તો સંતુલન તાપમાન ............ $\mathrm{K}$ હશે.View Solution
- 9$100\,^oC$ તાપમાને પ્રવાહી પાણીના બાષ્પમાં રૂપાંતર સાથે સંકળાયેલ એન્થાલ્પી/ફેરફાર $40.8\, kJ\, mol^{-1}$ છે. તો આ પ્રકમ માટે એન્ટ્રોપી ફેરફાર ....$J\,{K^{ - 1}}\,mo{l^{ - 1}}$ થશે.View Solution
- 10અચળ $T$ અને $P$ એ થતી અપ્રતિવર્તી પ્રક્રિયા કે જેમાં ફક્ત દબાણ-કદ પ્રકારનું કાર્ય થાય છે, તેના માટે ગિબ્સની મુક્તઊર્જા ફેરફાર $(dG)$ અને એન્ટ્રોપી ફેરફાર $(dS)$ કઇ શરતોનું પાલન કરે છે ?View Solution
