નીચેનામાંથી થરમૉડાયનેમિક્સનો ક્યો નિયમ આંતરિક ઉર્જા પદ ને વ્યાખ્યાયિત કરે છે ?
Easy
b
(b)
(b)
Internal energy is the sum of all the energies of a molecule in the frame of reference of the system.
According to \(1^{\text {st }}\) law,
\(\Delta Q=\Delta U+\Delta W\)
where \(\Delta U\) is internal energy, \(\Delta W\) is the work done and \(\Delta Q\) is the heat transferred.
\(\Delta U=\Delta Q-\Delta W\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
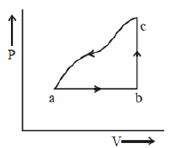
- 1એક બંધ ઓરડામાં $27^{\circ} \mathrm{C}$ તાપમાને રહેલો એક વાસ્તવીક વાયુ આક્રિત્તમાં દર્શાવ્યા અનુસાર ચક્રિય પ્રક્રિયાને અનુસરે છે. વાયુ પથ $A$ થી $B$ માટે $P V^3=R T$ સૂત્રને અનુસરે છે. એક પૂર્ણ ચક્ર દરમિયાન થતું કુલ કાર્ય ........... $J$ હશે. (Assuming $R=8 \mathrm{~J} / \mathrm{mol} \mathrm{K}$ )View Solution
- 2કાર્નોટ એન્જિન પહેલા $200^{\circ}\,C$ અને $0^{\circ}\,C$ વચ્ચે કાર્ય કરે છે અને પછી $0^{\circ}\,C$ અને $-200^{\circ}\,C$ વચ્ચે કાર્ય કરે છે. બંને કિસ્સામાં તેમની કાર્યક્ષમતાનો ગુણોત્તર $...............$View Solution
- 3$A$ અને $ B$ વાયુ સમાન દબાણ અને તાપમાને છે.તેનું સંકોચન કરી કદ $V$ થી $V/2$ કરવામાં આવે છે.$A$ નું સમતાપીય અને $B$ નું સમોષ્મી સંકોચન થાય છે.તો$A$ નું અંતિમ દબાણView Solution
- 4View Solutionસમોષ્મી અને સમતાપી પ્રક્રિયાના આલેખનો ઢાળનો ગુણોત્તર કેટલો થાય?
- 5નીચેના આલેખમાં થતુ કુલ કાર્ય $?$View Solution
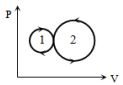
- 6આકૃતિમાં એક આદર્શ વાયુ માટે ચક્રિય પ્રક્રિયા $abca$ દર્શાવેલ છે.$ca$ પથ પર આંતરિક ઉર્જામાં થતો ફેરફાર $-180\, J$ છે.વાયુ $ab$ પથ પર $250\, J$ ઉષ્માનું શોષણ અને $bc$ પથ પર $60\, J$ ઉષ્માનું શોષણ કરે તો $abc$ પ્રક્રિયા દરમિયાન ..... $J$ કાર્ય થશે.View Solution
- 7એક થરમૉડાઇનેમિક પ્રક્રિયા દરમિયાન ચોક્કસ દળવાળા વાયુનું દબાણ એ રીતે બદલવામાં આવે છે કે જેથી વાયુના અણુઓ $20 J$ જેટલી ઉષ્મા ગુમાવે અને વાયુ પર $10 J$ જેટલું કાર્ય થાય છે. જો વાયુની પ્રારંભિક આંતરિક ઊર્જા $40 J$ હોય, તો અંતિમ આંતરિક ઊર્જા ...... $J$View Solution
- 8View Solutionનળાકાર પાત્રમાં રહેલ વાયુને પિસ્ટન દ્વારા સંકોચન કરવામાં આવે અને તેને તે જ સ્થિતિમાં રાખવામા આવે તો સમય જતાં ...
- 9View Solutionનીચેનામાંથી ક્યું રેફીજરેટરનું પરફોર્મન્સ ગુણાંક હોઈ શકે ?
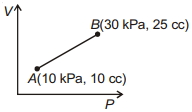
- 10આકૃતિમાં દર્શાવેલ પ્રક્રિયા માટે થયેલ કાર્ય .......... $J$ છે.View Solution