નિરપેક્ષ તાપમાન $T$ એ એક પાત્રમાં $N$ જેટલા અણુઓ રહેલા છે. પાત્રમાંની કુલ ગતિઊર્જા અચળ રહે તેમ અણુઓની સંખ્યા બમણી કરવામાં આવે તો નવું નિરપેક્ષ તાપમાન કેટલું થશે.
Medium
b
(b)
(b)
Initial energy of gas = Final energy
Let K.E. of each molecule initially be \(E_0\)
\(\therefore\) Total kinetic energy \(=E_0 \times n\)
Let final kinetic energy of each molecule be \(E_f\)
\(E_0 \times n=E_f \times 2 n\)
\(E_f=\frac{E_0}{2}\)
Since temperature is the average kinetic energy of molecules.
\(T_0=K E_0 \quad T_f=\frac{K E_0}{2}\)
\(\therefore\) Temperature becomes \(T_f=\frac{T_0}{2}\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1અચળ દબાણે આદર્શ વાયુના એક મોલ તાપમાનમાં $10 \;K$ વધારો કરવા માટે, $207\;J$ ઉષ્માની જરૂર પડે છે. જો સમાન વાયુનું તાપમાન અચળ કદ પર સમાન $10 \;K$ વધારવામાં આવે, તો જરૂરી ઊર્જા . ..... $J$ હશે. (આપેલ: વાયુ અચળાંક $R = 8.3\;J/mol-K$)View Solution
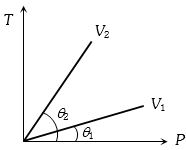
- 2View Solutionવાયુના જુદાં જુદાં કદ માટે તાપમાન વિરુધ્ધ દબાણનો આલેખ આપેલ છે,તો તેમના કદ વચ્ચેનો સંબંધ નીચે પૈકી ક્યો છે.
- 3એક બંધ પાત્રમાં એેક મોલ એક પરમાણ્વિક અને ત્રણમોલ દ્વિ પરમાણ્વિક વાયુનું મિશ્રણ ભરવામાં આવેલ છે. જો $R$ $=8 \,JK ^{-1} mol ^{-1}$ હોય તો અચળ કદે મોલર વિશિષ્ટ ઉષ્મા કેટલી થશે.View Solution
- 4અચળ દબાણે $27^°C$ તાપમાને રહેલા વાયુનું તાપમાન ....... $^oC$ કરવાથી $rms$ ઝડપ બમણી થાય.View Solution
- 5ખુલ્લા મોઢાવાળા પાત્રમાં $60°C$ એ હવા ભરવામાં આવે છે અને પાત્ર $T$ તાપમાને ગરમ કરવામાં આવે છે. જેથી હવાનો $1/4$ મો ભાગ બહાર નીકળી જાય છે તો $T$ ........ $^oC$ થાશે.View Solution
- 6$27°C$ તાપમાને આદર્શ વાયુની ગતિ ઊર્જા $E_1$ છે. જો તાપમાન $327°C$ વધારવામાં આવે તો ગતિ ઊર્જા શું થશે?View Solution
- 7${N}_{2}$ વાયુના અણુંની કયા તાપમાને ગતિઊર્જા $0.1\;volt$ થી પ્રવેગિત કરેલા સ્થિર ઇલેક્ટ્રોનની ગતિઊર્જા જેટલી થાય? (નજીકના પૂર્ણાંકમાં)View Solution
$\left(\right.\left.{k}_{{B}}=1.38 \times 10^{-23} \, {J} / {K}\right)$
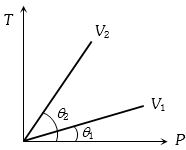
- 8View Solutionવાયુના જુદાં જુદાં કદ માટે તાપમાન વિરુધ્ધ દબાણનો આલેખ આપેલ છે,તો તેમના કદ વચ્ચેનો સંબંધ નીચે પૈકી ક્યો છે.
- 9View Solutionવિસ્તરણનો દર ...
- 10બે વાયુના અણુના દળ $M_1$ અને $M_2$ છે,તો સમાન તાપમાને તેના $rms$ ઝડપનો ગુણોત્તર કેટલો થાય?View Solution
