${C_{\left( {graphite} \right)}} + {O_{2\left( g \right)}} \to C{O_{2\left( g \right)}}\,;\,\Delta H = -393.5\,kJ$
${C_2}{H_{4\left( g \right)}} + 3{O_{2\left( g \right)}} \to 2C{O_{2\left( g \right)}} + 2{H_2}{O_{\left( l \right)}}\,;\,\Delta H = - 1410.9\,kJ$
${H_{2\left( g \right)}} + 1/2{O_{2\left( g \right)}} \to {H_2}{O_{\left( l \right)}}\,;\,\Delta H = - 285.8\,kJ$
\(i)\) \(C + O _2 \longrightarrow CO _2 \quad \Delta H _i=-393.5\)
\(ii)\) \(C _2 H _4+3 O _2 \longrightarrow 2 CO _2+2 H _2 O \quad \Delta H _{ ii }=-1410.9\)
\(iii)\) \(H _2+\frac{1}{2} O _2 \longrightarrow H _2 O \quad \Delta I _{ iii }=-25.8\)
\(iv)\) \(2 C +2 H _2 \longrightarrow C _2 H _4 \Delta H =(?)\)
\(iv)\) \(=2( i )-( ii )+2 (iii)\)
\(\therefore \Delta H =2 \Delta H _{ i }-2 \Delta H _{ ii }+2 \Delta H _{ iii }\)
\(=2(-393.5)-(-1410.9)+2(-285.8)\)
\(=-787+1410.9-571.6\)
\(=1410.9-1358.6\)
\(=52.3\, kJ\)
Download our appand get started for free
Similar Questions
- 1$45.0\, g$ સિલિકોનના તાપમાનમાં $6\,^oC$ નો વધારો કરવા $192\,J$ ઉષ્માની જરૂર પડે તો તેની વિશિષ્ટ ઉષ્માક્ષમતા.....View Solution
- 2$X \,gm$ ઈથેનાલ $(CH_3CHO)$ ને બોમ્બ કેલેરીમીટરમાં સળગાવે છે અને $Y$ જુલ ઉષ્મા ઉત્પન્ન થાય છે. તો .....View Solution
- 3બે સંયોજનો $x$ અને $y$ ની સર્જન એન્થાલ્પીના મૂલ્ય અનુક્રમે $-84\, kJ $ અને $-156 \,kJ$ છે,નીચેનામાંથી કયું વિધાન યોગ્ય છેView Solution
- 4પ્રક્રિયા માટેView Solution
$C _{2} H _{6} \rightarrow C _{2} H _{4}+ H _{2}$
પ્રકિયા એન્થાલ્પી $\Delta_{ r } H =...........{ kJ\, mol ^{-1}}$.
[આપેલ : બંધ એન્થાલ્પી $kJ$ $mol$ $^{-1}:C-C : 347, C = C : 611 ; C - H : 414, H - H : 436]$
- 5આપેલ :View Solution
$(i)\, {\Delta _f}{H^o}$ of $N_2O$ is $82\, kJ\, mol^{-1}$ છે,
$(ii)$ $N \equiv N,N = N,O = O$ અને $N = O$ બંધઊર્જા અનુક્રમે $946, 418, 498$ અને $607\, kJ\, mol^{- 1}$ છે. તો $N_2O$ ની સંસ્પંદન ઊર્જા ......$kJ$
- 6View Solutionઆદર્શ વાયુના ઉષ્મીય પ્રસરણ દરમ્યાન તેની.......
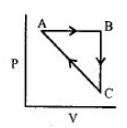
- 7એક આદર્શ વાયુ નીચે દર્શાવેલ આકૃતિ મુજબ ચક્રીય પ્રક્રમ અનુભવે છે.View Solution
$\Delta {U_{BC}} = - 5\,kJ\,mo{l^{ - 1}},{q_{AB}} = 2\,kJ\,mo{l^{ - 1}}$
$\Delta {W_{AB}} = - 5\,kJ\,mo{l^{ - 1}},{W_{CA}} = 3\,kJ\,mo{l^{ - 1}}$
$CA$ પ્રક્રમ દરમિયાન પ્રણાલી દ્વારા શોષાતી ઉષ્મા ......$kJ\,mo{l^{ - 1}}$
- 8$27^{\circ} \mathrm{C}$ પર અચળ કદે ધન બેન્ઝોઈક એસિડ ની દહનઉષ્મા $-321.30 \mathrm{~kJ}$ છે. અચળ દબાણ પર દહનઉષ્મા $(-321.30-x \mathrm{R}) \mathrm{kJ}$ છે. $x$ નું મૂલ્ય ......... છે.View Solution
- 9${I_{2\left( s \right)}}$ ની ઊર્ધ્વીકરણ ઊર્જા $57.3\, kJ\, mol^{-1}$ અને ગલન એન્થાલ્પી $15.5\, kJ\,mol^{-1}$ છે. તો ${I_2}$ ની બાષ્પાયન એન્થાલ્પી .....................$kJ\,mo{l^{ - 1}}$ થશે.View Solution
- 10નીચે આપેલા $p-V$ વક્રમાંથી ક્યું સૌથી વધારે થયેલ કાર્ય દર્શાવે છે?View Solution
