For the reaction,
$CaCO_{3(g)} \rightleftharpoons CaO_{(s)} + CO_{2(g)}$
$K_p = P_{CO_2}$ and $K_C = [CO_2]$
$(\because [CaCO_3] = 1$ and $[CaO] = 1$ for solids$)$
According to Arrhenius equation we have
$K = A{e^{ - \Delta H{^\circ _r}/RT}}$
Taking logarithm, we have
$\log {K_p} = \log\, A - \frac{{\Delta H_r^o}}{{RT(2.303)}}$
This is an equation of straight line. When $log \,K_p$ is plotted against $1 / T$. we get a straight line.
The intercept of this line = $ log \,A$, slope $= -\Delta H^°_r / 2.303 \,R$
Knowing the value of slope from the plot and universal gas constant $R$, $∆H^°_r$ can be calculated.
(Equation of straight line : $Y = mx + C$. Here,
$\log {K_p} = - \frac{{\Delta H_r^o}}{{2.303R}}\left( {\frac{1}{T}} \right) + \log A$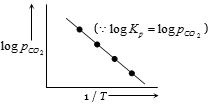
Download our appand get started for free
Similar Questions
- 1પ્રકિયા ${H_{2(g)}}\, + \,{I_{2(g)}}\, \rightleftharpoons 2H{I_{(g)}}$ માટે $440\,^oC$ તાપમાને $K_c$ નુ મૂલ્ય $50$ છે. જો પ્રક્રિયાની શરૂઆત $1\,L$ ની ફ્લાસ્કમાં $1$ મોલ $H_ 2$, $2$ મોલ $I_2$ અને $3$ મોલ $HI$ લઇને કરવામાં આવી હોય, તો $HI$ ની સંતુલન સાંદ્રતા .......... $M$ થશે.View Solution
- 2નીચેની પ્રક્રિયા માટે વાયુમય તબક્કામાં .........${K_p}/{K_c}$.View Solution
$CO\left( g \right) + \frac{1}{2}{O_2}\left( g \right) \to C{O_2}\left( g \right)$
- 3View Solutionરાસાયણિક સંતુલન સ્વભાવમાં ગતિશીલ છે કારણ કે
- 4પ્રક્રિયા $CuSO_4.5H_2O_{(s)} $ $\rightleftharpoons$ $ CuSO_4.3H_2O_{(s)} + 2H_2O_{(g)}$ માટે નીચેનામાંથી કયું એક સાચું નિરૂપણ દર્શાવે છે ?View Solution
- 5પ્રકિયા $3A + {\text{ B}}\,\, \rightleftharpoons \,\,2C\, + \,D$ માટે જો $A,\,B$ અને $C$ ની સંતુલન સાંદ્રતાઓ અનુક્રમે $0.03,\, 0.01\,M$ અને $0.008$ હોય, તો $A$ ની શરૂઆતની સાંદ્રતા શું થશે ?View Solution
- 6નીચે આપેલી પ્રતિવર્તી રાસાયણીક પ્રક્રિયા ધ્યાનમાં લો.View Solution
${A_2}(g)\, + \,{B_2}(g)\,\overset {{K_1}} \leftrightarrows \,2AB(g)\,\,\,......(1)$
$6AB\,(g)\,\,\overset {{K_2}} \leftrightarrows \,\,3{A_2}(g)\, + \,3{B_2}(g)......(2)$
તો $K_1$ અને $K_2$ વચ્ચેનો સંબંધ શું થાય?
- 7$1990\, K$ અને $1\, atm$ દબાણ પર, પ્રક્રિયા મિશ્રણમાં $Cl _{2}$ અણુઓ અને $Cl$ પરમાણુઓ સમાન સંખ્યામાં છે. $Cl _{2( g )}=2 Cl _{( g )}$ પ્રક્રિયા માટે ઉપરની પરિસ્થિતિઓ હેઠળ $K_p$ નું મૂલ્ય $x \times 10^{-1}$ છે. તો $x$ નું મૂલ્ય .............. છે. (નજીકના પૂર્ણાકમાં રાઉન્ડ ઑફ)View Solution
- 8પ્રક્રિયા $A + 2B$ $ \rightleftharpoons $ $2C + D$ માં $B$ ની શરૂઆતની સાંદ્રતા $[A]$ કરતા $1.5$ ગણી છે પરંતુ સંતુલને $A$ અને $B$ ની સાંદ્રતાઓ સમાન બને છે. તો પ્રક્રિયા માટે સંતુલન અચળાંક ગણોView Solution
- 9સંતુલન પર, સાંદ્રતાView Solution
$N_{2}=3.0 \times 10^{-3} M$
$O_{2}=4.2 \times 10^{-3} M$
અને $N O=2.8 \times 10^{-3} M$
આપેલ પ્રક્રિયા માટે બંધ કરેલા વાસણમાં $800 \,K$ અને $1$ $atm$ દબાણે $K_{p}$ ......... $atm$ હશે ?
$N_{2}(g)+O_{2}(g) \rightleftharpoons 2 N O(g)$
- 10$400\, K$ તાપમાને $20\, litre$ નુ પાત્ર $0.4$ વાતા. દબાણે $CO_{2(g)}$ અને પૂરતા પ્રમાણમાં $SrO$ ધરાવે છે. (ઘન $SrO$ નું કદ અવગણો) હવે પાત્રમાં ફિટ કરેલા સરકી શકે તેવા પિસ્ટનને ખસેડી પાત્રનું કદ ઘટાડવામાં આવે છે. જ્યારે $CO_{2(g)}$ નુ દબાણ તેનુ મહત્તમ મૂલ્ય પ્રાપ્ત કરે ત્યારે પાત્રનું મહતમ કદ કેટલા ......લિટર થશે ?View Solution
(અહીં : $SrCO_{3(s)} \rightleftharpoons SrO_{(s)}+ CO_{2(g)} \,, K_p=1.6\,atm$)
