શન્ય ક્રમની પ્રક્રિયા માટે વેગ અચળાંકનો એકમ ..........થશે.
AIPMT 2011, Easy
a
The rate law expression for the zero order reaction is Rate $= k [ A ]^0$
The rate law expression for the zero order reaction is Rate $= k [ A ]^0$
$mol\,L ^{-1}\, \sec ^{-1}= k \times\left( mol\,L ^{-1}\right)^0$
Unit of $k = mol\, L ^{-1}\, sec ^{-1}$
The unit of rate constant for a zero order reaction is $mol\, L ^{-1} \,s ^{-1}$.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચેની પ્રક્રિયા $xA \longrightarrow yB$ માં $“A”$ અને $“B”$ અનુક્રમે શુ હોઇ શકે ?View Solution
${\log _{10}}\,\left[ { - \frac{{d\left[ A \right]}}{{dt}}} \right] = {\log _{10}}\,\left[ {\frac{{d\left[ B \right]}}{{dt}}} \right] + 0.3010$
- 2દ્વિતીય ક્રમ પ્રક્રિયા માટે કે જેના બંને પ્રક્રિયકો સમાન પ્રારંભિક સાંદ્રતા ધરાવે છે અને પ્રક્રિયા $20\%$ પુરી થવા માટે $500$ સેકન્ડ લાગે છે. તો પ્રક્રિયાને $80\%$ પુરી થવા ......... સેકન્ડ લાગશે.View Solution
- 3રેડીયોએક્ટિવ સમસ્થાનીકનું અર્ધઆયુ ત્રણ કલાક છે. જો સમસ્થાનિકનું શરૂઆતનું દળ $256\, g$ હોય તો $18$ કલાક પછી કેટલા $g$ દળ બાકી રહેશે?View Solution
- 4દ્વિતીય ક્રમની પ્રક્રિયા માટે જેમાં બંને પ્રક્રિયકોની પ્રારંભિક સાંદ્રતા સમાન હોય છે. પ્રક્રિયાને $60\%$ પૂર્ણ થવા માટે $3000$ સેકન્ડનો સમય લાગશે. તો પ્રક્રિયાનો $20\%$ પુરી થવા ....... $\sec$ લાગે.View Solution
- 5નીચે આપેલ દર અચળાંક $(k)$ સાથે તાપમાન $(T)$ વચ્ચેનો તફાવત આલેખમાં દર્શાવ્યો છે. તો કયો આલેખ આર્હેંનિયસ સમીકરણનું પાલન કરે છે.View Solution
- 6જો પ્રથમ ક્રમની પ્રક્રિયા માટે $T_1 $ તાપમાને વેગ અચળાંક $K_1 $ તથા તાપમાન $T_2 $ એ વેગઅચળાંક $K_2 $ હોય, તો નીચેનામાંથી કયો સંબંધ સાચો છે.? ( $E_a $ = સક્રિયકરણ ઉર્જા)View Solution
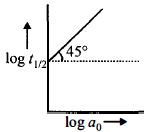
- 7પ્રક્રિયા $A \to$ નીપજો માટે $log\,t_{1/2}$, વિરુદ્ધ $log\,a_0$ નો આલેખ આકૃતિમાં દર્શાવ્યો છે. જો $A$ ની શરૂઆતની સાંદ્રતા $a_0,$, વડે દર્શાવવામાં આવે તો પ્રક્રિયાની ક્રમ જણાવો.View Solution
- 8$H_2 + I_2 $ $\rightleftharpoons$$H_I$ પ્રક્રિયા માટે સાચો સંબંધ લખો.View Solution
- 9View Solutionઅસરકારક અથડામણ માટે અથડામણ સમયે પ્રક્રિયક અણુઓ પાસે જે ન્યૂનતમ ઊર્જા હોવી જોઇએ તેને ... કહે છે.
- 10View Solutionધનાત્મક ઉદ્દીપક પ્રક્રિયા માટે સાચી પ્રક્રિયા પ્રોફાઈલ આલેખ (ડાયાગ્રામ) શોધો.
