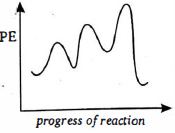
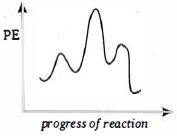
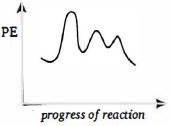
ત્રણ પગલાની પ્રક્રિયા માટે ઉર્જાના આલેખની આકૃતિ દોરો જેમાં પ્રથમ પગલું સૌથી ધીમું અને છેલ્લું પગલું સૌથી ઝડપી છે. (ધારો કે પ્રકિયા ઉષ્માશોષક છે )
Medium
c
Acc. to Arrehenious equation \(K = Ae^{-E_a/RT}\)
Acc. to Arrehenious equation \(K = Ae^{-E_a/RT}\)
\(\therefore\) greater is \(E_a\), the smaller is \(K\) i. e., rate of reaction \(\propto \, \frac{1}{height\, of \,energy\, barrier}\)
It should be noted that step leading to the height peak will not be rate determining step if the concentration used in some other step is sufficiency high. Since first step is rate determining step.
\(\therefore\) First peak should be high as is \((c)\).
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$87.5 \%$ પ્રક્રિયા પૂર્ણ થવા માટે જરૂરી સમય $t_{87.5}$ છે અને $50 \%$ પ્રક્રિયા પૂર્ણ થવા માટે જરૂરી સમય $t _{50}$ છે. $t _{87.5}=x \times t _{50}$ સંબંધ ધરાવે છે.તો $x$ નું મૂલ્ય $........$ છે.View Solution
- 2View Solutionઉદીપક એ પ્રક્રિયાનો વેગ........... વધારશે.
- 3View Solutionપ્રથમ ક્રમ પ્રક્રિયાનું સાચુ વિધાન ....... થશે.
- 4$25\,C$ એ પ્રથમ ક્રમ પ્રક્રિયાની સક્રિયકરણ ઊર્જા $30\,kJ/$ મોલ છે. તો તે જ પ્રક્રિયાની $25\,^oC $ એ ઉદ્દીપકની હાજરીમાં તેની સક્રિયકરણ ઊર્જા $24\,kJ/$ મોલ છે. તો ઉદ્દીપકની હાજરીમાં પ્રક્રિયાનો દર પહેલા કરતા ........ ગણો થશે.View Solution
- 5$2 NO ( g )+ Cl _{2}( g ) \rightleftharpoons 2 NOCl ( s )$View Solution
આ પ્રક્રિયાનો $-10^{\circ} C$ પર અભ્યાસ કરાયો હતો અને નીચેની માહિતી મળી હતી.
ક્રમ $[ NO ]_{0}$ $\left[ Cl _{2}\right]_{0}$ $r _{0}$ $1$ $0.10$ $0.10$ $0.18$ $2$ $0.10$ $0.20$ $0.35$ $3$ $0.20$ $0.20$ $1.40$ $[ NO ]_{0}$ અને $\left[ Cl _{2}\right]_{0}$ શરૂઆતની સાંદ્રતા અને $r _{0}$ શરૂઆતનો પ્રક્રિયાનો વેગ છે, તો પ્રક્રિયાનો ક્રમ શું હશે?
- 6ઉચ્ચ ક્રમની $ ( > 3) $ પ્રક્રિયાઓ વિરલ છે કારણ કે :View Solution
- 7પ્રક્રિયા $2FeC{l_3} + SnC{l_2} \to 2FeC{l_2} + SnC{l_4}$ શેનું ઉદાહરણ છે?View Solution
- 8એક ફ્લાસ્ક સંયોજનો $AB$ અને $XY$ નુ મિશ્રણ ધરાવે છે.તેઓને ગરમ કરતા બંનેનુ વિઘટન પ્રથમ કમની પ્રકિયા મુજબ થાય છે. જો $AB$ અને $XY$ ના અર્ધઆયુષ્ય સમય અનુક્રમે $30\,\min$ અને $10\,\min$ હોય, તો $AB$ ની સાંદ્રતા $XY$ ની સાંદ્રતા કરતા ચાર ગણી થતા ....... $\min.$ લાગશે. ($AB$ અને $XY$ ની શરૂઆતની સાંદ્રતા સમાન ગણો)View Solution
- 9ઓઝોનને ગરમ કરવાથી તેનુ ઓક્સિજનમાં નીચે મુજબ વિધટન થાય છે.View Solution
${O_3} \rightleftharpoons {O_2} + \left[ O \right]$
${O_3} + \left[ O \right] \to 2{O_2}$ (slow)
તો $2{O_3} \to 3{O_2}$ પ્રક્રિયાનો કમ જણાવો.
- 10View Solutionપ્રથમક્રમ પ્રક્રિયાનો વિશિષ્ટ દર અચળાંક ....... પર આધારિત છે.