ઉષ્માશોષક પ્રક્રિયા માટે, સક્રિયકરણની ઊર્જા $E_a$ છે અને પ્રક્રિયાની ઊર્જા એ $\Delta H$ (આ બંને $kJ/mol$ માં) છે.$E_a$નું ન્યૂનતમ મૂલ્ય હશે?
AIPMT 2010, Medium
c
In endothermic reactions, energy of reactants is less than that of the products. Potential energy diagram for endothermic reactions is
In endothermic reactions, energy of reactants is less than that of the products. Potential energy diagram for endothermic reactions is
\(E_{a}= E_{a}^{\prime}+\Delta H\)
where, \(E_{a}=\) activation energy of forward reaction
\(E_{a}^{\prime}=\) activation energy of backward reaction
\(\Delta \mathrm{H}=\) enthalpy of the reaction.
From the diagram below,
\(E_{a}= E_{a}^{\prime}+\Delta H\)
\(E_{a}>\Delta H\)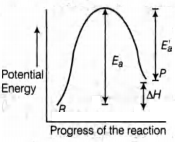
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1એક પ્રક્રિયાનો વેગ $r=K[x]\, [y]/[OH^-]$ છે. જો $[OH^-]$ વધારે હોય, તો પ્રક્રિયાકમ ........ થશે.View Solution
- 2$50\,mm$ $AB_3$ નું ઉદ્દીપકીય વિઘટન માટે અદ્ય આયુ સમય $4$ કલાક અને $100\,mm$ એ તેને $2$ કલાક લાગે છે તો પ્રક્રિયાનો ક્રમ..... થશે?View Solution
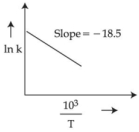
- 3$700 -$ $1000\,K$ તાપમાન શ્રેણીમાં (વિસ્તાર માં) એસિટાલ્ડીહાઈડના વિઘટન માટેના દર (વેગ) અચળાંક માપવામાં આવ્યાં. $\ln k$ વિરુદ્ધ $\frac{10^{3}}{ T }$ આલેખ દોરીને માહિતીનું પૃથ્થકરણ કરવામાં આવ્યું.પ્રક્રિયા માટે સક્રિયકરણ શક્તિનું મૂલ્ય $\dots\dots\dots$$kJ\,mol { }^{-1}$ છે.(નજીકનો પૂર્ણાંક)View Solution
(આપેલ:$R =8.31\,JK ^{-1}\,mol ^{-1}$)
- 4વાયુરૂપ પ્રક્યિા ${A_{\left( g \right)}} \to 2{B_{\left( g \right)}} + {C_{\left( g \right)}}$ પ્રથમ ક્રમની પ્રક્રિયા છે. જો પ્રક્રિયાની શરૂઆતમાં $P_A = 90\, mm\, Hg$ હોય અને $10\, min$ બાદ કુલ દબાણ $180\, mm\, Hg$ જણાય, તો પ્રક્રિયાનો વેગઅચળાંક જણાવો.View Solution
- 5$C_2H_5I + OH \rightarrow C_2H_5OH + I^{-}$ પ્રક્રિયા માટે $30^o$ સે. અને $60^o$ સે. તાપમાને વેગ અચળાંકના મલ્યો અનુક્રમે $0.325$ અને $6.735$ લિટર મોલ$^{-1}$ સેકન્ડ $^{-1} $ હોય તો સક્રિયકરણ ઊર્જા $(E_a)$ નું મૂલ્ય .......... કેલરી થશે.View Solution
- 6પ્રક્રિયકો $A$ અને $B$ ને સમાવતી પ્રક્રિયાનો વેગ = $= k[A ]^n[B]^m$ છે. જો A ની સાંદ્રતા બમણી અને B ની સાંદ્રતા અડધી કરીએ તો તવા વેગ અને મૂળ વગનો ગુણોત્તર ......... થશે.View Solution
- 7બે જુદી-જુદી પ્રક્રિયા માટે વેગ અચળાંક $k_1$ અને $k_2$ અનુક્રમે $10^{16} \cdot e^{-2000/T}$ અને $ 10^{15} \cdot e^{-1000/T}$ છે. તો ક્યાં તાપમાને $k_1 = k_2$ થશે ?View Solution
- 8પ્રક્રિયા $2FeC{l_3} + SnC{l_2} \to 2FeC{l_2} + SnC{l_4}$ શેનું ઉદાહરણ છે?View Solution
- 9View Solutionઆલ્કલાઇન માધ્યમમાં એસ્ટરનું જળ વિભાજન .......
- 10સંકલિત દર સમીકરણ $Rt = \log \;{C_0} - \log {C_t}$ છે તે સીધો ગ્રાફ કયા ઢાળદ્વારા પ્રાપ્ત થાય છેView Solution
