વિધાન : જ્યારે ગરમ દૂધ ભરેલા ગ્લાસને રૂમમાં ઠંડો કરવા મૂકવામાં આવે તો તેની એન્ટ્રોપી ઘટે.
કારણ : ગરમ વસ્તુને ઠંડા કરવામાં થર્મોડાયનેમિકના બીજા નિયમનું ઉલંઘન થતું નથી.
AIIMS 2006, Easy
b
A body cools, its entropy decreases as \(dS = \frac {dQ}{T}\) and \(dQ\) is \(-ve, dS\) is also \(-ve\). \(R\) is also true. Second law states that entropy of the universe increases. Universe includes both system and surroundings. \(R\) does not explain \(A\).
A body cools, its entropy decreases as \(dS = \frac {dQ}{T}\) and \(dQ\) is \(-ve, dS\) is also \(-ve\). \(R\) is also true. Second law states that entropy of the universe increases. Universe includes both system and surroundings. \(R\) does not explain \(A\).
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$P$ દબાણે અને $V$ કદે રહેલ એક એક પરમાણ્વીય વાયુ અચળાંક જ સંકોચન અનુભવે છે અને તેનું ક્દ ઘટીને મૂળ કદ કરતા આઠમાં ભાગનું થઈ જાય છે. અચળ એન્ટ્રોપી એ અંતિમ દબાણ $.....P$ હશે.View Solution
- 2વાતાવરણ દબાણે અને $273\, K$ તાપમાને રહેલ બરફ ઓગળે તો....View Solution
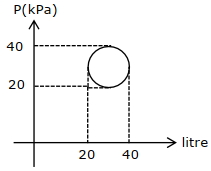
- 3નીચે આપેલી ચક્રીય પ્રક્રિયામાં કેટલી ઉષ્માનું ($\pi J$ માં) શોષણ થશે?View Solution
- 4${27^o}C$ તાપમાને રહેલ હિલિયમનું કદ $8$ લિટર છે.તેનું અચાનક સંકોચન કરીને કદ $1$ લિટર કરતાં વાયુનું તાપમાન ....... $^oC$ થાય? $[\gamma = 5/3]$View Solution
- 5એક રેફ્રિજરેટરનું અંદરનું તાપમાન $t_2\, ^o C$ છે અને ઓરડાનું તાપમાન $t_1 \,^o C$ છે. આદર્શ અવસ્થામાં પ્રતિજૂલ વિદ્યુતઊર્જાનો વ્યય થાય ત્યારે, ઓરડાને આપેલી ઉષ્માનું મૂલ્ય કેટલું હશે?View Solution
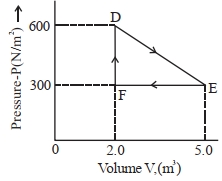
- 6એક થર્મોડાયનેમિક તંત્રને આકૃતિમાં દર્શાવ્યા અનુસાર તેની પ્રારંભિક અવસ્થા $D$ માંથી વચ્ચેની અવસ્થા $E$ માં એક રૈખીય પ્રક્રિયાથી લઈ જવામાં આવે છે. ત્યારબાદ તેનું કદ મૂળ કદ જેટલું ધટાડવામાં આવે છે અને તે સમદાબીય પ્રક્રિયા દ્વારા $E$ થી $F$ જાય છે. વાયુ દ્વારા $D$ થી $E$ થી $F$ જતાં થતું કુલ કાર્ય $..........\,J$ હશે.View Solution
- 7કોઈ એક પ્રક્યિામાં એક મોલ એક પરમાણ્વીક આદર્શ વાયુના કદ અને તાપમાનમાં $ VT=K$ ના સબંધ અનુસાર બદલાય છે. જ્યાં $K$ એ અચળાંક છે. આ પ્રક્રિયામાં વાયુના તાપમાનને $\Delta T$ જેટલું વધારવામાં આવે છે. વાયુ દ્વારા શોષાતી ઊષ્માનો જથ્થો કેટલો હશે. ($R$ વાયુ અચળાંક છે).View Solution
- 8અવાહક પાત્રમાં $4 \,mol$ આદર્શ દ્વિ પરમાણ્વિક વાયુ $T$ તાપમાને ભરેલ છે.વાયુને $Q$ ઉષ્મા આપતાં $2\, mol$ વાયુનું એક પરમાણ્વિક વાયુમાં રૂપાંતર થાય છે.જો તાપમાન અચળ રહેતું હોય,તો ઉષ્મા $Q$ કેટલી હશે?View Solution
- 9એક કિલોમોલ વાયુનું સમોષ્મી સંકોચન કરવા માટે $146 kJ $ કાર્ય કરવામાં આવે છે. આ પ્રક્રિયા દરમિયાન વાયુનું તાપમાન $7 °C$ જેટલું વધે છે. આ વાયુ ........ છે.View Solution
- 10View Solutionથરર્મોડાયનેમિક પ્રક્રિયા માટે કયું વિધાન ખોટું છે.?
