$27\,^oC $ એ, $36\,g$ ગ્લુકોઝ પ્રતિ લીટરમાં અભિસરણ દબાણ $4.92 $ વાતાવરણ છે. જો સમાન તાપમાને દ્રાવણનું અભિસરણ દબાણ $1.5$ વાતાવરણ કરવામાં આવે તો તેની સાંદ્રતા કેટલી થાય?
Diffcult
b
આપેલ, $\pi_1 = 4.92\,atm$ , $\pi_2 = 1.5\,atm$
આપેલ, $\pi_1 = 4.92\,atm$ , $\pi_2 = 1.5\,atm$
$\,{C_1}\,\, = \,\,\frac{{36}}{{180\,\, \times \,\,1}}$
$\left( {\because \,\,C\,\, = \,\,\frac{w}{{m\,\, \times \,\,V}}} \right)\,\,\,{C_2}\,\, = \,\,?$
$\pi_1V_1 = n_1S_1T_1$ અને $\pi_2V_2 = n_2ST_2$ સમાન તાપમાનેે,
$\frac{{{\pi _1}}}{{{\pi _2}}}\,\, = \,\,\frac{{{n_1}}}{{{n_2}}}\,\, \times \,\,\frac{{{V_2}}}{{{V_1}}}\,\, = \,\,\frac{{{C_1}}}{{{C_2}}}$ અથવા $\frac{{4.92}}{{1.5}}\,\, = \,\,\frac{{36}}{{180\,\, \times \,\,{C_2}}}$
$\therefore \,\,{C_2}\,\, = \,\,0.061\,\,mol/litre$
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionજેમાંથી એકમાં સૌથી વધુ અભિસરણ દબાણ હોય છે
- 2$18\,g$ ગ્લુકોઝ $(C_6H_{12}O_6)$ ને $178.2\, g$ પાણીમાં ઉમેરવામાં આવેલ છે. તો આ જલીય દ્રાવણનું બાષ્પદબાણ $100\,{\,^o}C$ એ ($torr$ માં) શોધો.View Solution
- 3બાષ્પદબાણમાં $75\%$ નો ઘટાડો કરવા $114\,g$ ઓક્ટેનમાં દ્રાવ્ય કરવા પડતા અબાષ્પશીલ, વિધુતઅવિભાજ્ય દ્રાવ્ય (મોલર દળ $= 50\,g\,mol^{- 1})$ નુ દળ ........... $\mathrm{g}$ જણાવો.View Solution
- 4${H_2}S{O_4}$ દ્રાવણની મોલારિટી $......$ $M$ છે, જેની $35\,^oC$ પર ઘનતા $1.84$ ગ્રામ$ / cc$ છે અને વજન અનુસાર $98\%$ દ્રાવ્ય સમાવે છે.View Solution
- 5$25\,^oC $ સે. એ $CCl_4$ નું બાષ્પ દબાણ $143\,\,mm\,Hg$. $0.5\,gm$ $100\,ml$ $CCl_4$ માં અબાષ્પશીલ દ્રાવ્યને (અ.ભા. $65$ ) દ્રાવ્ય કરવામાં આવે છે તો દ્રાવણનું બાષ્પદબાણ કેટલા ........... $\mathrm{mm}$ હશે? ( $CCl_4$ ની ઘનતા $= 1.58 \,\,gm/cm^3$)View Solution
- 6સાંદ્ર સક્યુરિક એસીડ વ્યાપારી ધોરણે વજનથી $95\%\,\,H_2SO_4$ તરીકે મળે છે. જો આ વ્યાપારીક એસિડની ધનતા $1.834\,g\,cm^{-3},$ હોય તો આ દ્રાવણની મોલારિટી જણાવો.View Solution
- 7$12\,g$ યુરિયા $1$ લિટર પાણીમાં અને $68.4\,g$ સુક્રોઝને $1$ લિટર પાણીમાં ઓગળવામાં આવે છે.પ્રથમ કિસ્સામાં બાષ્પ દબાણ શું હશે ?View Solution
- 8View Solutionઅબાષ્પશીલ દ્રાવ્ય માટે રાઉલ્ટના નિયમ મુજબ .........
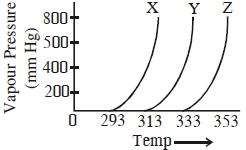
- 9ત્રણ જુદા જુદા પ્રવાહીઓ $X, Y$ અને $Z$ માટે બાષ્પદબાણ અને તાપમાનનો આલેખ નીચે દર્શાવ્યો છે. નીચેના તારણો કરવામાં આવ્યા.View Solution
(A)$Y$ ની સરખામણીમાં $\mathrm{X}$ માં આંતરઆણ્વિય આંતરક્રિયા વધુ છે
(B)$Y$ ની સરખામણીમાં $\mathrm{X}$ માં આંતરઆણ્વિય આંતરક્રિયા ઓછી છે
(C)$Y$ ની સરખામણીમાં $\mathrm{Z}$ માં આંતરઆણ્વિય આંતરક્રિયા ઓછી છે
સાચું તારણ(ણો) જણાવો.
- 10યુરિયાના $10\,g$ દીઠ $dm^3$ (અણુ સમૂહ $= 60\,g\,mol^{-1}$ ) નો સમાવિષ્ટ એક આબાષ્પશીલ દ્રાવકના $5\%$ દ્રાવણ સાથે આઇસોટોનિક છે તો આબાષ્પશીલ દ્રાવક નું આણ્વિય દળ ........ $g\,mol^{-1}$View Solution
