એક હવાના પરપોટા (bubble)નું કદ બમણુંં થઈ જાય છે, જ્યારે તે તળાવના તળિયેથી તેની સપાટી સુધી ઉપર ઊઠે છે. વાતાવરણનું દબાણ પારાનું $75 \,cm$ છે. પારાથી તળાવના પાણીની ઘનતાનો ગુણોત્તર $\frac{40}{3}$ છે તો તળાવની ઉંચાઈ મીટરમાં કેટલી છે ?
Medium
a
(a)
(a)
\(2 P_0=P_0+\rho g h\)
\(\Rightarrow P_0=\rho g h\)
\(\Rightarrow P_0=75 \,cm \text { mercury } \quad \text { [Atmospheric pressure] }\)
\(\Rightarrow \rho_{\text {mercury }} \times g \times \frac{75}{100}=\rho_{\text {water }} \times g \times h\)
\(\Rightarrow \frac{\rho_m}{\rho_w} \times \frac{75}{100}=h\)
\(\Rightarrow \frac{40}{3} \times \frac{75}{100}=h \quad\left[\because \frac{\rho_m}{\rho_w}=\frac{40}{3} \text { (given) }\right]\)
\(\Rightarrow h=10 \,m\)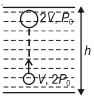
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
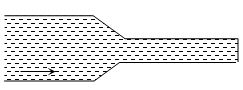
- 1હીરાના એક જ સ્ફટિકમાંથી આકૃતિમાં દર્શાવ્યા મુજબના આકારનું એરણ (anvils) બનાવેલ છે. તેનો ઉપયોગ ઊંચા દબાણ હેઠળ દ્રવ્યની વર્તણૂક તપાસવા માટે થાય છે. એરણના સાંકડા છેડા પાસે સપાટ બાજુઓના વ્યાસ $0.50\, mm$ છે. જો એરણના પહોળા છેડાઓ પર $50,000\, N$ નું દાબીય બળ લાગુ પાડેલ હોય, તો એરણના સાંકડા છેડે (tip) દબાણ કેટલું હશે.View Solution
- 2${m_1}$દળ અને${s_1}$ વિશિષ્ટ ઘનતા ધરાવતા પદાર્થને${m_2}$દળ અને${s_2}$ વિશિષ્ટ ઘનતા ધરાવતા પદાર્થ સાથે મિશ્રણ કરવાથી મિશ્રણની વિશિષ્ટ ઘનતા કેટલી થાય?View Solution
- 3એક હાઈડ્રોલીક પ્રેસ $100\, kg$ ને ઊંચકી શકે છે જ્યારે $‘m'$ જેટલું દળ નાના પિસ્ટન પર મૂકવામાં આવે છે. દળ ને $‘m’$ જેટલું સમાન રાખીને જો મોટા પીસ્ટનનો વ્યાસ $4$ ગણો વધારવામાં આવે અને નાના પીસ્ટનનો વ્યાસ $4$ ગણો ઘટાડવામાં આવે તો તે ............... $kg$ દળ ઊંચકી શકશે.View Solution
- 4સ્પ્રે પમ્પના નળાકારની ટયૂબની ત્રિજયા $R$ છે, તેના એક છેડે $r$ ત્રિજયાના $n$ સૂક્ષ્મ છિદ્રો છે. જો ટયૂબમાં પ્રવાહીની ઝડપ $v$ હોય, તો આ છિદ્રોમાંથી બહાર નીકળતા પ્રવાહીની ઝડપ કેટલી હશે?View Solution
- 5બે એકસમાન નળાકાર પાત્રને જમીન પર મૂકેલા છે જેમાં સમાન ઘનતા $d$ ધરાવતું પ્રવાહી ભરેલ છે. બને પાત્રના તળિયાનું ક્ષેત્રફળ $S$ છે પરંતુ એક પાત્રમાં પ્રવાહીની ઊંચાઈ $x_{1}$ અને બીજા પાત્રમાં પ્રવાહીની ઊંચાઈ $x_{2}$ છે. જ્યારે બંને નળાકારને નહિવત કદ ધરાવતી નળી દ્વારા પાત્રના તળીએથી જોડવામાં આવે છે જેથી જ્યાં સુધી બંને પાત્રમાં પ્રવાહી એક નવી ઊંચાઈના સંતુલનમાં ના આવે ત્યાં સુધી પ્રવાહી એક પાત્રમાંથી બીજા પાત્રમાં વહન કરે છે. આ પ્રક્રિયા દરમિયાન તંત્રની ઊર્જામાં કેટલો ફેરફાર થાય?View Solution
- 6એક અસમાન વ્યાસ ધરાવતી નળી માથી પ્રવાહી ધારારેખી રીતે વહન કરે છે.નળીનો મહત્તમ અને ન્યુનત્તમ વ્યાસ અનુક્રમે $6.4 \;\mathrm{cm}$ અને $4.8 \;\mathrm{cm}$ છે, તો પ્રવાહીના ન્યુનત્તમ અને મહત્તમ વેગનો ગુણોત્તર કેટલો થાય?View Solution
- 7સ્ટોક્સના નિયમની સકાચણી કરવા માટે કરેલા પ્રયોગમાં $r$ ત્રિજ્યા અને $\rho$ ઘનતા ધરાવતા એક ગોળ દડાને પાણી ભરેલા પાત્રમાં પાણીની સપાટીથી $h$ ઊંચાઈ પરથી મુક્ત કરવામાં આવે છે. જો પાણીની અંદર દડાનો ટર્મિનલ વેગ એ પાણીની અંદર આવતા પહેલા દડાના વેગ જેટલો હોય તો ઊંચાઈ $h$ કોના સમપ્રમાણમાં હશે? (હવાનો શ્યાનતાગુણાંક અવગણો)View Solution
- 8એક નિયમિત આડ-છેદની શિરોલંબ $U-$ટ્યૂબએે બંને ભૂજામાં પાણી ધરાવે છે. કોઈ પણ એક ભૂજા પર $10 \,cm$ ની ગ્લિસરીન સ્તંભ ઉમેરવામાં આવે છે. ($R.D. = 1.2$) બંને ભૂજામાં બંને મુક્ત સપાટીઓ વચ્ચેના સ્તરનું તફાવત ........ $cm$ હશે ($R.D =$ સાપેક્ષ ધનતા)View Solution
- 9$r$ ત્રિજયા અને ધનતા ધરાવતો ગોળો $ h$ ઊંચાઇ પરથી મુકત કરતાં,તે પાણીમાં પડે ત્યારે ટર્મિનલ વેગ પ્રાપ્ત કરે છે.જો પાણીનો શ્યાનતા ગુણાંક $\eta$ હોય,તો $h=$View Solution
- 10View Solutionઆકૃતિમાં દર્શાવેલ નળીમાં પ્રવાહીનું વહન થાય છે.તો દબાણ વિરુધ્ધ અંતરનો આલેખ મેળવો ?