$N{H_{3(g)}}\, + \,\,\frac{3}{2}\,Cu{O_{(s)}}\, \to \,\,\frac{1}{2}\,{N_{2(g)}}\, + \,\,\frac{3}{2}{H_2}{O_{(\ell )}}\, + \,\,\frac{3}{2}\,C{u_{(s)}}.$ ......$J$
\(\,N{H_{3(g)}}\, + \,\,3/2Cu{O_{(s)}}\, \to \,\,1/2{N_{2(g)}}\,\, + \,\,3/2{H_2}{O_{(l)}}\, + \,\,3/2\,C{u_{(s)}}\)
\(\Delta \,H\,\, = \,\,\Sigma \,\,{H_{\ Products}}\,\, - \,\,\,\Sigma \,{H_{\operatorname{Reactants}}}\)
\(\because \,\,H_{element}^ \circ \,\, = \,\,0\)
\(\therefore \,\,\Delta \,H\,\, = \,\,\frac{3}{2}\,H_{{H_2}O}^o \,\, - \,\,H_{N{H_3}}^o \, - \,\,\frac{3}{2}\,H_{CuO}^o \)
\( = \,\,\frac{3}{2}\,\, \times \,\,( - 285)\, - \,( - 46)\, - \,\,\frac{3}{2}\,( - 155)\,\,\,\,\,\,\,\,\,\,\, = \,\, - 149\,\,KJ\)
\(\because \,\,17\,\,gm\,\,N{H_3}\) ના ફેરફાર જોતાં \(\Delta \,\,H\,\, = \,\, - \,149\,\,KJ\)
\(\,6.8\,\,gm\,\,N{H_3}\,\) ના ફેરફાર જોતાં \(\Delta H\,\, = \,\, - \frac{{149}}{{17}}\,\, \times \,\,6.8\,\,KJ\)
\( = \,\, - 59.6\,\,KJ\,\,\,\,\,\,\,\,\,\,\,\Delta \,H\,\, = \,\, - \,\,59.6\,\,KJ\)
Download our appand get started for free
Similar Questions
- 1View Solutionસમોષ્મી પ્રકમ ................. હોય છે.
- 2$300\, {~K}$ પર આપેલ કેમિકલ $A \rightarrow B$ માટે મુક્ત ઊર્જા ફેરફાર $-49.4\, {~kJ} \,{~mol}^{-1}$ અને પ્રક્રિયાની એન્થાલ્પી $51.4\, {~kJ} \,{~mol}^{-1}$ છે. પ્રક્રિયાનો એન્ટ્રોપી ફેરફાર $.....\,{J}\, {K}^{-1}\, {~mol}^{-1}$ છે.View Solution
- 3નીચેના પૈકી માર્ગવિધેય (path function) રજૂ કરતો માપદંડોનો સેટ જણાવો.View Solution
$(a)\,\,q + w$ $ (b)\,\,q$
$(c)\,\,w$ $ (d)\,\,H -TS$
- 4એક પ્રક્રિયા માટે $\ln K_{eq}$ વિરુદ્ધ તાપમાનના વ્યસ્તનો આલેખ નીચે મુજબ છે. તો પ્રક્રિયા ........ હશે.View Solution
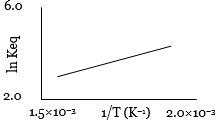
- 5$400\, mL$ $0.2\, M$ $H_2SO_4$ ને $600\, mL$ $0.1\, M$ $NaOH$ સાથે મિશ્ર કરવામાં આવે ત્યારે ઉત્પન્ન થતી ઉષ્માનો જથ્થો $3.43\, kJ$ છે. જો પાણીની વિશિષ્ટ ઉષ્મા $4.18\, J\, K^{-1}\,g^{-1}$ હોય, તો અંતિમ દ્રાવણના તાપમાનમાં થતો વધારો ...........$K$ થશે. (અંતિમ દ્રાવણની વિશિષ્ટ ઉષ્માક્ષમતા પાણી જેટલી ધારો)View Solution
- 6તબક્કા પરિવર્તન માટે, ${H_2}O(l)\underset {{0^{\,o}}C,\,1\,bar} \longleftrightarrow {H_2}O(s)$View Solution
- 7અચળ $T$ અને $P$ અપ્રતિવર્તીં પ્રક્રિયા થાય છે. જેમાં માત્ર દબાણ-કદના કાર્ય દ્વારા ગીબ્સ મુક્ત ઉર્જા ($\Delta G$) માં ફેરફાર થાય છે. કઈ પરિસ્થિતિમાં એન્ટ્રોપી ફેરફાર ($\Delta S$) સંતોષકારક છે ?View Solution
- 8View Solutionએન્ટ્રોપી એટલે .......
- 9સ્વયંભૂ પ્રક્રિયા માટે $\Delta G$ સંતુલન અચળાંક $(K)$ અને $E^o_{cell}$ શું થાય?View Solution
- 10$H_2, Cl_2$ અને $HCl$ માટે બંધ વિયોજન એન્થાલ્પીના મૂલ્યો અનુક્રમે $434,242$ અને $ 431 kJ/mol$ છે. તો $HCl$ ની સર્જન એન્થાલ્પી ............. $\mathrm{kJ \,mol}^{-1}$View Solution
