કાર્નોટ એન્જિનની કાર્યક્ષમતા $100\%$ હોતી નથી,કારણ કે
AIEEE 2002, Medium
c
(c) The efficiency of Carnot engine is \(\quad \eta=1-\frac{T_{2}}{T_{1}}\)
(c) The efficiency of Carnot engine is \(\quad \eta=1-\frac{T_{2}}{T_{1}}\)
where, \(T_{1}\) is the temperature of the source and \(T_{2}\) that
of sink. since, \(\frac{T_{2}}{T_{1}}=\frac{Q_{2}}{Q_{1}}\)
So, \(\quad \eta=1-\frac{Q_{2}}{Q_{1}} |\)
To obtain \(100 \%\) efficiency (i.e., \(\eta=1), Q_{2}\) must be zero i.e., if a sink at absolute zero would be available, all the heat taken from the source would have been converted into work. The temperature of sink means a negative temperature on the absolute scale at which the efficiency of engine is greater than unity. This would be a violation of the \(2\, nd\) law of thermodynamics. Hence, a negative temperature on the absolute scale is impossible. Hence, we cannot reach absolute zero temperature.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
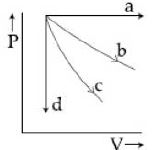
- 1View Solutionઆપેલ ગ્રાફમાં ચાર પ્રક્રિયા આપેલ છે સમકદ,સમદાબી,સમતાપી અને સમોષ્મિ પ્રક્રિયાનો સાચો ક્રમ નીચેનામાથી કયો થશે?
- 2$T$ તાપમાને રહેલ એક $R$ ત્રિજયાના પોલા ગોળાને ધ્યાનમાં લો. તેની અંદર રહેલા કાળા-પદાર્થ વિકિરણને,જેની એકમ કદ દીઠ આંતરિક ઊર્જા $E=$ $\frac{U}{V} \propto {T^4}$ અને દબાણ $P = \frac{1}{3}\left( {\frac{U}{V}} \right)$ ધરાવતા ફોટોનના બનેલા આદર્શ વાયુ તરીકે વિચારી શકાય. હવે જો આ પોલો ગોળો જો સમોષ્મી વિસ્તરણ અનુભવે તો $T$ અને $R$ વચ્ચેનો સંબંધ:View Solution
- 3સમોષ્મી પ્ર્ક્રિયા દરમ્યાન, વાયુનું દબાણ તેના નિરર્પેક્ષ તાપમાનના ઘનના સમપ્રમાણમાં માલૂમ પડે છે, તો વાયુ માટે $\frac{C_P}{C_V}$ ગુણોત્તર. . . . . . . .હશે.View Solution
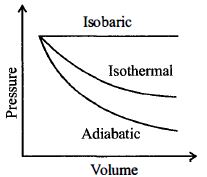
- 4આપેલ વાયુ નું વિસ્તરણ $V_1$ થી $V _2$ અલગ અલગ પ્રક્રિયા દ્વારા કરવામાં આવે તો કઈ પ્રક્રિયામાં સૌથી વધુ કાર્ય થાય?View Solution
- 5પાત્રમાં રહેલ આદર્શ વાયુને પિસ્ટન દ્વારા અલગ કરવામાં આવે છે,એક ભાગની એન્ટ્રોપી $S_{1}$ અને બીજા ભાગની એન્ટ્રોપી $S_{2}$ છે, $S _{1}> S _{2}$ જો પિસ્ટનને દૂર કરવામાં આવે તો તંત્રની કુલ એન્ટ્રોપી શું થશે?View Solution
- 6$3.00$ મોલ આદર્શ દ્વિ પરમાણ્વિક વાયુનું તાપમાન દબાણ અચળ રાખીને $40.0^{\circ} {C}$ જેટલું વધારવામાં આવે છે. વાયુના અણું ચાકગતિ કરે છે પરંતુ કંપન કરતાં નથી. જો આંતરિકઉર્જાનો ફેરફાર અને વાયુ દ્વારા થતાં કાર્યનો ગુણોત્તર $\frac{{x}}{10}$ છે. તો ${x}$ નું મૂલ્ય (નજીકના પૂર્ણાંકમાં) કેટલું હશે? $\left(\right.\left.{R}=8.31\, {J} {mol}^{-1} {K}^{-1}\right)$View Solution
- 7View Solutionસમોષ્મી પ્રક્રિયા માટે બલ્ક મોડયુલસ આદર્શ વાયુ માટે કેટલો હોય?
- 8સમતાપીય ફેરફારમાં વાયુના દબાણ અને કદના ફેરફાર ત્રણ ભિન્ન તાપમાન $T_3 >T_2 >T_1$ માટે નીચે મુજબ રજૂ કરવામાં આવે છે.View Solution
- 9રેફીજરેટરના બહારના ભાગનું અને અંદરના ભાગનું તાપમાન અનુક્રમે $273 \,K$ અને $300 \,K$ છે. ધારો કે રેફ્રીજેરટરનું યક્ર પ્રતિવર્તી છે, થયેલ કાર્યની દરેક જૂલ માટે, પરિસરમાં આપવામાં આવતી ઉષ્મા લગભગ ......... $J$ હશે.View Solution
- 10કાર્નોટ એન્જિન $400\, K$ અને $800\, K$ વચ્ચે કાર્ય કરે છે. ચક્ર દીઠ કાર્ય $1200\, J$ હોય તો ચક્ર દીઠ એન્જિનને અપાતી ઉષ્મા .......... $J$ હશે.View Solution
