ઉષ્મીય રીતે અલગ પાડેલ એક પાત્ર $M$ પરમાણુભાર અને $\gamma$ જેટલો વિશિષ્ટ ઉષ્માઓનો ગુણોત્તર ધરાવતો આદર્શ વાયુ ધરાવે છે. તે $v$ ઝડપથી ગતિ કરી રહ્યો છે અને અચાનક તેને રોકવા આવે છે. આસપાસ ઉષ્માનો કોઇ વ્યય થતો નથી એમ ધારતા તેના તાપમાનમાં થતો વધારો કેટલો હશે?
AIEEE 2011, Medium
c
If \(m\) is the total mass of the gas then its kinetic energy\( = \frac{1}{2}m{v^2}\)
If \(m\) is the total mass of the gas then its kinetic energy\( = \frac{1}{2}m{v^2}\)
When the vessel is suddenly stopped then total kinetic energy will increase the temperature of the gas. Hence
\(\frac{1}{2}m{v^2} = \mu \,{C_v}\Delta T\)\( = \frac{m}{M}{C_v}\Delta T\) [As \({C_v} = \frac{R}{{\gamma - 1}}\)]
==>\(\frac{m}{M}\frac{R}{{\gamma - 1}}\Delta T = \frac{1}{2}m{v^2}\) ==> \(\Delta T = \frac{{M{v^2}(\gamma - 1)}}{{2R}}\).
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$20\,L$ કદનું પાત્રએ $27 ^{\circ}\,c$ તાપમાન અને $2\,atm$ દબાણે હાઈડ્રોજન અને હિલિયમનું મિશ્રણ ધરાવે છે.મિશ્રણનું દળ $5\,g$ નું છે. જો વાયુને આદર્શ ગણવામાં આવે, તો, હાઈડ્રોજન અને હિલિયમના દળનો ગુણોતરView Solution
- 2$T$ તાપમાને રહેલ આદર્શ વાયુમાં બંધ પાત્રની દીવાલ પર અણું દ્વારા લાગતું સરેરાશ બળ $T^q$ પર આધાર રાખે છે. તો $q$ નું મૂલ્ય કેટલું હશે?View Solution
- 3વિધાન : વાયુની $rms$ ઝડપ અને મહતમ શક્ય ઝડપ સમાન હોયView Solution
કારણ : વાયુની ઝડપ માટે મેક્સવેલ ગ્રાફ સમમિતિ ધરાવે છે.
- 4$T$ તાપમાને અને $P$ દબાણે $32$ ગ્રામ $O _2$ એક પાત્રમાં ભરવામાં આવ્યો છે. એેવા જ પાત્રમાં $2T$ તાપમાને $4$ ગ્રામ $H _2$ ભરવામાં આવે તો દબાણ કેટલું હશે.View Solution
- 5આણ્વિક વ્યાસ $d$ અને અંકઘનતા $n$ ધરાવતા એક વાયુના સરેરાશ મુક્ત પથને ........... વડે રજૂ કરી શકાય છેView Solution
- 6$T$ તાપમાન માટે એક પરમાણ્વિક વાયુ માટે $\bar v , \bar v_{rms}$ અને $v_p$ અનુક્રમે સરેરાશ ઝડપ, $rms$ ઝડપ અને મહત્તમ શક્ય ઝડપ છે. અણુનું દળ $m$ હોય તો .....View Solution
- 7એક આદર્શ વાયુના અણું પાસે ત્રણ રેખીયગતિના મુક્તતાના અંશો અને બે ચાકગતિના મુક્તતાના અંશો છે. વાયુને $T$ તાપમાને રાખેલ છે.આ વાયુની કુલ આંતરિક ઉર્જા $U$ અને $\gamma\left(=\frac{ C _{ P }}{ C _{ v }}\right)$ ના મૂલ્યો કેટલા થશે?View Solution
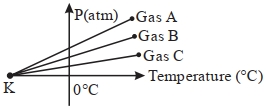
- 8આકૃતિમાં દર્શાવ્યા અનુસાર ત્રણ નિમ્ન ઘનતા ધરાવતા વાયુઓ $A,B,C$માટે તેમનું કદ અચળ રહે તે સ્થિતિમાં: દબાણ વિરુદ્ધ તાપમાનના આલેખો દોરેલા છે.બિંદુ $K$ ને અનુરૂપ તાપમાન $.........\,{}^{\circ}\,C$ થશે.View Solution
- 9પાત્રમાં $m$ દળ ધરાવતા $N$ અણુઓ વાયુ $A$ ના અને $2m$ દળ ધરાવતા $2N$ અણુઓ વાયુ $B$ ના ભરેલા છે.વાયુ $A$ ના x-ઘટકના વેગના વર્ગનો સરેરાશ $ {w^2} $ અને વાયુ $B$ નો વેગના વર્ગનો સરેરાશ $ {v^2} $ હોય,તો $ \frac{{{w^2}}}{{{v^2}}} $View Solution
- 10એક અવાહક દિવાલવાળા વાયુપાત્રમાં બે ચેમ્બર બનાવવામાં સાવી છે. બંને ચેમ્બરને એક અવાહક દિવાલથી અલગ પાડવામાં આવે છે. પ્રથમ ચેમ્બરનું ક્ $V _{1}$ છે અને તેમાં $P _{1}$ દબાણવાળો અને $T _{1}$ તાપમાનવાળો આદર્શવાયુ ભરેલો છે. બીજી ચેમ્બરનું કદ $V _{2}$ છે અને તેમાં $P _{2}$ દબાણવાળો અને $T _{2}$ તાપમાનવાળો આદર્શવાયુ ભરેલો છે. જો વાયુ પર કોઈ પણ પ્રકારનું કાર્ય કર્યા સિવાય, વચ્ચેની દીવાલ દૂર કરવામાં આવે, તો તંત્ર સંતુલન સ્થિતિ પ્રાપ્ત કરે ત્યારનું તાપમાન કેટલું થાય?View Solution
