બે મોલ દ્વિ પરમાણ્વિક આદર્શ વાયુનું સમોષ્મી સંકોચન કરી તેનું કદ $50\%$ કરવા $830\, J$ કાર્ય કરવું પડે છે.તેના તાપમાનમા થતો ફેરફાર ....... $K$ હશે? $(R\, = 8.3\, J\,K^{-1}\, mol^{-1} )$
JEE MAIN 2014, Medium
c
\(Given:Work\,done,W=830J\)
\(Given:Work\,done,W=830J\)
no, of moles of gas, \(\mu =2\)
For diatomic gas \(\gamma = 1.4\)
Work done during an adiabatic change
\(W = \frac{{\mu R\left( {{T_1} - {T_2}} \right)}}{{\gamma - 1}}\)
\( \Rightarrow 830 = \frac{{2 \times 8.3\left( {\Delta T} \right)}}{{1.4 - 1}} = \frac{{2 \times 8.3\left( {\Delta T} \right)}}{{0.4}}\)
\( \Rightarrow \Delta T = \frac{{830 \times 0.4}}{{2 \times 8.3}} = 20\,K\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1વાયુનો નમૂનો $V_1$ કદથી $V_2$ કદમાં વિસ્તરે છે. વાયુ દ્વારા થતું કાર્ય મહતમ હોય જ્યારે વિસ્તરણ .......... હોય.View Solution
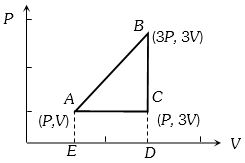
- 2નીચે આપેલી ચક્રીય પ્રક્રિયા $ABCA$ માં કેટલું કાર્ય થાય?View Solution
- 3$18^oC$ રહેલા તાપમાને દ્વિ પરમાણ્વિક વાયુનું સમોષ્મી સંકોચન કરતાં કદ મૂળથી આઠમાં ભાગનું થાય છે. સંકોચન પછી તાપમાન કેટલું થાય?View Solution
- 4View Solutionનીચે આપેલા કયા તાપમાન માટે કાર્નોટ એન્જિનની કાર્યક્ષમતા મહત્તમ હોય.
- 5કાર્નોટ એન્જિન $ {411^o}C $ અને $ {69^o}C $ વચ્ચે કાર્ય કરે છે.તેને ચક્રદીઠ અપાતી ઉષ્મા $1000 \,J$ હોય,તો ચક્ર દીઠ ....... $J$ કાર્ય થશે?View Solution
- 6એક દ્વિ-પરમાણ્વીક વાયુ $(\gamma=1.4)$ નું જ્યારે સમદાબીય રીતે વિસ્તરણ કરવામાં આવે છે ત્યારે તે $400\,J$ કાર્ય કરે છે. આ પ્રક્રિયામાં વાયુને આપવી પડતી ઉષ્મા .......... $J$ છે.View Solution
- 7આદર્શ વાયુનું કદ $1 $ લીટર છે તથા તેનું દબાણ $72 \,\,cm$ પારાના દબાણ જેટલુ છે તેને સમતાપી રીતે દબાવીને તેનું કદ $900 \,\,cm^{3}$ કરવામાં આવેલ છે તો ગેસનો પ્રતિબળ.... $ cm$ (પારાનું) ?View Solution
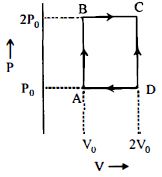
- 8$n$ મોલ આદર્શ વાયુ એન્જિન $ABCD$ પથ પર ચક્રિય પ્રક્રિયા કરે છે તો એન્જિનની ઉષ્મા કાર્યક્ષમતા કેટલી થાય? ($C_v =1 .5\, R$, $R-$ વાયુ અચળાંક)View Solution
- 9જો સમોષ્મી પ્રક્રિયા માટે $\gamma = 2.5$ તથા કદ તેના પ્રારંભીક કદ કરતા $1/8 $ ગણું હોય તો દબાણ $P' =.... $ (પ્રારંભીક દબાણ $= P$)View Solution
- 10View Solutionસમતાપી પ્રક્રિયા માટે કયું વિધાન ખોટું છે.
