since the reaction is $2\,nd$ order w.r.t $CO$. Thus, rate law is given as.
$r=k[C O]^{2}$
Let initial concentration of $CO$ is a i.e. $[C O]=a$
$r_{1}=k(a)^{2}=k a^{2}$
when concentration becomes doubled, i.e. $[\mathrm{CO}]=2 a$
$\therefore r_{2}=k(2 a)^{2}=4\, k a^{2}$
$\therefore \quad r_{2}=4 r_{1}$
So, the rate of reaction becomes $4\,times$ .
Download our appand get started for free
Similar Questions
- 1મોટા ભાગની પ્રક્રિયાનો તાપમાન ગુણાંક $.......$ ની વચ્ચે હોય છે.View Solution
- 2પ્રક્રિયાની શ્રેણીમાં $A\,\xrightarrow{{{K_1}}}\,\,\,B\,\,\,\xrightarrow{{{K_2}}}\,\,\,C\,\,\,\xrightarrow{{{K_3}}}\,\,\,D\,\,;\,\,{K_3}\,\,\, > \,\,\,{K_2}\,\,\, > \,\,{K_1},$તો પ્રક્રિયાનો દર ક્યા તબબકા વડે નક્કી થશે ?View Solution
- 3View Solutionજો પ્રક્રિયકની પ્રારંભિક સાંદ્રતા બમણી કરતા અર્ધઆયુષ્ય અડધું થશે તો પ્રક્રિયાનો ક્રમ શું થશે?
- 4પ્રથમક્રમની પ્રક્રિયા માટે શરૂઆતની સાંદ્રતામાં $1/4$ જેટલો ઘટાડો થવા માટે લાગતો સમય $20$ મિનિટ છે. તો શરૂઆતની સાંદ્રતા માં $1/16 $ જેટલો ઘટાડો થવા માટે લાગતો સમય......... $\min.$ હશે.View Solution
- 5દ્વિતીય ક્રમ પ્રક્રિયા માટે કે જેના બંને પ્રક્રિયકો સમાન પ્રારંભિક સાંદ્રતા ધરાવે છે અને પ્રક્રિયા $20\%$ પુરી થવા માટે $500$ સેકન્ડ લાગે છે. તો પ્રક્રિયાને $80\%$ પુરી થવા ......... સેકન્ડ લાગશે.View Solution
- 6નીચેના વિશેષો માટે પ્રથમ ક્રમના તત્વો સાથે પ્રથમ વર્તુળ પ્રક્રિયા વિશેષોમાં માન્ય રેક્ટિવ હોય છે, જેમાં સ્થિર તાપમાન છે.View Solution
$\mathrm{A}+\mathrm{B} \underset{\text { Step } 3}{\text { Step } 1} \mathrm{C} \xrightarrow{\text { Step } 2} \mathrm{P}$
પ્રથમના વર્તુળ પ્રક્રિયાની માહિતી નીચે સૂચવેલી છે.
સ્ટેપ Rate constant $\left(\sec ^{-1}\right)$
Activation energy
$\left(\mathrm{kJ} \mathrm{mol}^{-1}\right)$
$1$ ${k}_1$ $300$ $2$ ${k}_2$ $200$ $3$ ${k}_3$ $\mathrm{Ea}_3$ ઉપરોક્ત રીતેની પ્રક્રિયાનું વધારણીક વર્તુળ $(k)$ આપવામાં આવે છે. $\mathrm{k}=\frac{\mathrm{k}_1 \mathrm{k}_2}{\mathrm{k}_3}$ અને ઉપરોક્ત વધારણીક તાપ $(E_2)= 400$ કેલ્વિન છે, તો $\mathrm{Ea}_3$ નું મૂલ્ય છે $\mathrm{kJ} \mathrm{mol}^{-1}$ (નજીકની પૂર્ણાંક).
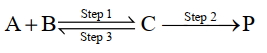
- 7$H_2 + I_2 $ $\rightleftharpoons$$H_I$ પ્રક્રિયા માટે સાચો સંબંધ લખો.View Solution
- 8View Solutionનીચે પૈકી કયું વિધાન સાચું છે?
- 9આર્ગોનની હાજરીમાં $N_2O$ નુ $N_2$ અને $O_2$ માં વિઘટન પ્રથમ કમની ગતિકીને અનુસરે છે. $K = 5.0 \times10^{11}\,e^{-2000/T}$ હોય તો પ્રકીયા સક્રિયકરણ ઊર્જા થશે?View Solution
- 10View Solutionએસ્ટરનું એસીડીક જળ વિભાજન....... છે.
