સાચી પરિસ્થિતિ માટે સાચી થર્મોડાયનેમિક પ્રક્રિયા પસંદ કરો. આપેલ ટેબલમાં $\Delta Q$ એ આપેલ ઉષ્મા, $\Delta W$ એ કાર્ય અને $\Delta U$ એ તંત્રની આંતરિક ઉર્જામાં થતો ફેરફાર છે.
| પ્રક્રિયા | પરિસ્થિતિ |
| $(I)$ સમોષ્મી | $(A)\; \Delta W =0$ |
| $(II)$ સમતાપી | $(B)\; \Delta Q=0$ |
| $(III)$ સમકદ | $(C)\; \Delta U \neq 0, \Delta W \neq 0 \Delta Q \neq 0$ |
| $(IV)$ સમદાબી | $(D)\; \Delta U =0$ |
JEE MAIN 2020, Medium
a
\((I)\) Adiabatic process \(\Rightarrow \Delta Q=0\) No exchange of heat takes place with surroundings
\((I)\) Adiabatic process \(\Rightarrow \Delta Q=0\) No exchange of heat takes place with surroundings
\((II)\) Isothermal proess \(\Rightarrow\) Temperature remains constant \((\Delta T =0)\)
\(\Delta u =\frac{ F }{2} nR \Delta T \Rightarrow \Delta u =0\)
No change in internal energy \([\Delta u =0]\)
\((III)\) Isochoric process Volume remains constant
\(\Delta V =0\)
\(W =\int P \cdot d V =0\)
Hence work done is zero.
\((IV)\) Isobaric process \(\Rightarrow\) Pressure remains constant
\(W = P . \Delta V \neq 0\)
\(\Delta u =\frac{ F }{2} nR \Delta T =\frac{ F }{2}[ P \Delta V ] \neq 0\)
\(\Delta Q = n C _{ p } \Delta T \neq 0\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1એક દ્વિ-પરમાણ્વીક વાયુ $(\gamma=1.4)$ નું જ્યારે સમદાબીય રીતે વિસ્તરણ કરવામાં આવે છે ત્યારે તે $400\,J$ કાર્ય કરે છે. આ પ્રક્રિયામાં વાયુને આપવી પડતી ઉષ્મા .......... $J$ છે.View Solution
- 2નળાકારમાં રાખેલા એક મોલ હીલીયમને કુલ $48 \mathrm{~J}$ઉષ્મા આપવામાં આવે છે. હીલીયમ વાયુનું તાપમાન $2^{\circ} \mathrm{C}$ જેવુ વધે છે. વાયુ દ્વારા થતું કાર્ય. . . . . .થશે. $( \mathrm{R}=8.3 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$ આપેલ છે.)View Solution
- 3કાર્નોટ એન્જિનના ઠારણ-વ્યવસ્થાનું તાપમાન $27°C$ છે અને કાર્યક્ષમતા $25\%$ છે, તો ઉષ્મા-પ્રાપ્તિસ્થાનનું તાપમાન ...... $^oC$View Solution
- 4View Solutionબે જુદી જુદી થર્મોડાયનેમિક પ્રક્રિયા માટે કયા ગ્રાફ સાચા છે?
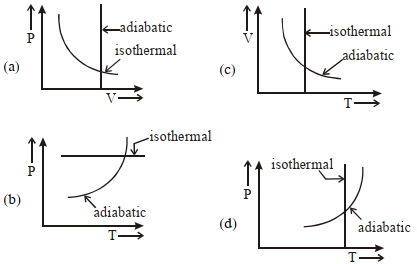
- 5View Solutionકાર્નોટ ચક્રમાં ......... નો સમાવેશ થાય છે.
- 6વાયુનું સમોષ્મી સંકોચન કરીને કદ અડધું કરતાં તાપમાન $ \sqrt 2 $ ગણું થાય છે.તો નીચેનામાથી કયું સમીકરણ સાચું છે.View Solution
- 7પાણીના ઠારણબિંદુ અને ઉત્કલનબિંદુ વચ્ચે કાર્ય કરતાં એક આદર્શ ઉષ્માયંત્રની કાર્યક્ષમતા ($\%$ માં) કેટલી થાય?View Solution
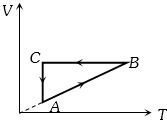
- 8ચક્રીય પ્રક્રિયા $ABCA$ નોકદ વિરુધ્ધ તાપમાનનો આલેખ આપેલ છે,તો દબાણ વિરુધ્ધ કદનો આલેખ ક્યો થશે?View Solution
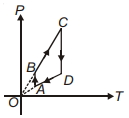
- 9ચક્રિય પ્રક્રિયા માટે $P-T$ આલેખ દર્શાવેલ છે. આને અનુરૂપ સાચું નિવેદન પસંદ કરો...View Solution
- 10$100\, ^{\circ}C$ તાપમાને રહેલ $1\,kg$ પાણીને પ્રમાણિત દબાણે $100^{\circ}\,C$ તાપમાને રહેલ વરાળમાં રૂપાંતરિત કરવામાં આવે છે. પાણીનું કદ $1.00 \times 10^{-3}\,m ^3$ થી વરાળમાં $1.671\,m ^3$ થાય છે.આ પ્રક્રિયામાં તંત્રની આાંતરિક ઊર્જાને ફેરફાર લગભગ $........\,kJ$ થશે. (બાષ્પાયન ગુપ્ત ઉષ્મા = $2257\,kJ / kg$ આપેલ છે,વાતાવરણનું દબાણ = $\left.1 \times 10^5\,Pa \right)$View Solution
