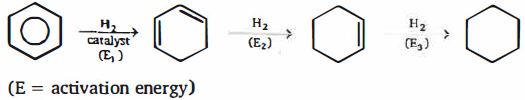Question Bank
Explore our large set of questions to practice for your standard seamlessly- 1View Solutionકયું ન્યૂનત્તમ ઉત્કલનબિદુ ધરાવે છે ?
- 2View Solutionકયું સંયોજન ઍરોમેટિક નથી?
- 3View Solutionકયો આલ્કીન સૌથી સ્થિર હોય છે ?
- 4કયો પ્રક્રિયક પ્રોપીનમાંથી $1$-પ્રોપેનોલમાં રૂપાંતર કરે છે ?View Solution
- 5View Solutionકેરોસીન એ......... નું મિશ્રણ છે.
- 6View Solutionકેલ્શિયમ કાર્બાઇડની પાણી સાથેની પ્રક્રિયાથી કઇ નીપજ મળે છે.
- 7View Solutionકેલ્શિયમ કાર્બાઇડની ભારે પાણી સાથેની પ્રક્રિયાથી શું મળે છે ?
- 8View Solutionકોલટાર એ.... નો મુખ્ય સ્ત્રોત છે.
- 9View Solutionજંતુનાશક ગેમેક્સિનનું રાસાયણિક નામ..... છે.
- 10View Solutionજળવાયુએ......... નું મિશ્રણ છે.
- 11જ્યારે જલદ $HNO_3$ અને $H_2SO_4$ ના મિશ્રણની બેન્ઝિન સાથે $353 \,K$ તાપમાને પ્રક્રિયા થાય ત્યારે તે પ્રક્રિયા કયા નામથી ઓળખાય છે ?View Solution
- 12જ્યારે બેન્ઝિનનું $FeCl_3$, ની હાજરીમાં ક્લોરિનેશન કરવામાં આવે છે ત્યારે ઇલેકટ્રોન અનુરાગી પ્રક્રિયક ....... છે.View Solution
- 13View Solutionટેટ્રાઇથાઇલ લેડનો ઉપયોગ ............... તરીકે થાય છે.
- 14View Solutionટોલ્યુઇનનું નાઇટ્રેશન....... સ્થાનમાં થાય છે.
- 15View Solutionનાઇટ્રિક એસિડ અને સલ્ફયુરિક એસિડ દ્વારા થતી બેન્ઝિનની નાઇટ્રેશન પ્રક્રિયા એ........ પ્રક્રિયાનું ઉદાહરણ છે.
- 16View Solutionનાઇટ્રોસમૂહની બેન્ઝિન ચક્રમાં હાજરીએ બેન્ઝિન ચક્રને.........
- 17View Solutionનીચેના પૈકી આકારની ર્દષ્ટિએ સમતલીય સંયોજન કયું છે ?
- 18View Solutionનીચેના પૈકી કયો બંધ ધરાવતું સંયોજન સૌથી વધુ સક્રિય છે ?
- 19View Solutionનીચેના પૈકી.......... હાઇડ્રોકાર્બન નથી
- 20View Solutionનીચેનામાંથી એરોમેટિક કોણ નથી ?
- 21View Solutionનીચેનામાંથી કયું એક સૌથી વધુ ક્રિયાશીલ છે જે ઈલેકટ્રો નાઈટ્રેશન તરફ રહે છે ?
- 22નીચેનામાંથી કયો આલ્કીન ઉદ્દીપકીય હાઈડ્રોજીનેશન સ્થિતિમાં $H_2 $ સાથે ઝડપી પ્રક્રિયા કરે છે ?View Solution
- 23View Solutionનીચેનામાંથી કયો એરોમેટીક પદાર્થ છે ?
- 24View Solutionનીચેનામાંથી કયો પ્રક્રિયક ઍસિડિક ગુણધર્મ ધરાવે છે ?
- 25View Solutionનીચેનામાંથી કયો હેલોજન અને આલ્કેન સાથે વિસ્ફોટક પ્રક્રીયા કરશે ?
- 26View Solutionનીચેના સંયોજનોમાં એવું સંયોજન જે સહેલાઇથી સલ્ફોનેશન કરી શકાય છે તે ....... છે.
- 27View Solution........ નો ઉપયોગ ફ્રિડલ ક્રાફટસ પ્રક્રિયામાં થતો નથી.
- 28View Solutionપેટ્રોલમાં આઇસો-ઓક્ટેનનો ઉપયોગ ............ માટે થાય છે.
- 29View Solutionપેરોકસાઈડની હાજરીમાં કયો હાઈડ્રોજન હેલાઈડ એ પ્રતિ (એન્ટિ) માર્કોવનિકોફ નિયમ પ્રમાણે પ્રોપીન સાથે યોગશીલ પ્રક્રિયા આપે છે ?
- 30પ્રક્રિયા $C_6H_6 \rightarrow C_6H_5CH_3$ …….View Solution
- 31View Solutionપ્રક્રિયા કઇ છે ?
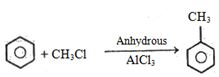
- 32View Solutionપ્રવાહી એમોનિયામાં સોડિયમ સાથે કયો હાઈડ્રોકાર્બન પ્રક્રિયા કરી શકે છે ?
- 33View Solutionપ્રાથમિક આલ્કેનથી તૃતીયક આલ્કેન તરફ જતાં ઉત્કલનબિંદુ.......
- 34View Solutionપ્રોપિનમાંથી પ્રોપેન કઇ પદ્ધતિ દ્વારા બનાવી શકાય છે ?
- 35પ્રોપીનની $HOCl $ સાથેની પ્રક્રિયા એ......યોગશીલ દ્વારા થાય છે ?View Solution
- 36View Solutionફ્રિડલ ક્રાફટસ પ્રક્રિયાઓમાં......... ઉદ્દીપક તરીકે વપરાય છે.
- 37ફ્રિડલ ક્રાફટસ પ્રક્રિયામાં નિર્જળ $AlCl_3$ નું કાર્ય ........ છે.View Solution
- 38View Solutionફ્રિડલ-ક્રાફટ્સ પ્રક્રિયામાં કયા પ્રક્રિયકો વાપરી શકતા નથી ?
- 39View Solutionફ્રિડલ-ક્રાફટ્સ પ્રક્રિયામાં ટોલ્યુઇન...... વડે બનાવી શકાય.
- 40View Solution......... બંધ સૌથી વધુ એસિડિક છે.
- 41View Solutionબેન્ઝિનના સલ્ફોનેશનમાં વપરાતો ઇલેક્ટ્રોન અનુરાગી ઘટક..........છે.
- 42બેન્ઝિનમાં $1,3$ સ્થાન શેના તરીકે ઓળખાય છે ?View Solution
- 43View Solutionબેન્ઝિનમાં કાર્બન-કાર્બન બંધનો બંધ ક્રમાંક.......
- 44બેન્ઝિનમાં રહેલા સીગ્મા બંધ અને પાઇ $(\pi)$ બંધની સંખ્યા અનુક્રમે ....... છે.View Solution
- 45બેન્ઝોઇક એસીડને $X$ સાથે અથવા ફિનોલ ને $Y$ સાથે ગરમ કરતા બેન્ઝિન મળે છે તો $X$ અને $Y$ અનુક્રમે......View Solution
- 46View Solutionબેયરનો પ્રક્રિયક ....
- 47બ્યુટ $-1-$ આઇનમાં એસિડિક હાઇડ્રોજનની સંખ્યા ........View Solution
- 48બ્યુટ-$1$-આઇનમાં એસિડિક હાઇડ્રોજનની સંખ્યા...... છે.View Solution
- 49બ્યુટા-$1,2$-ડાઇમાં રહેલા કાર્બન પરમાણુના સંકરણો........ પ્રકારના છે.View Solution
- 50View Solutionબ્યુટીનમાંથી બ્યુટેન........ સાથેની પ્રક્રિયા દ્વારા બની શકે છે.
- 51View Solutionમાર્શગેસનો મુખ્ય ઘટક......... છે.
- 52View Solutionમુક્ત મુલકનો નિર્માણ થવાનો ક્રમ કયો છે ?
- 53View Solution......... વાયુ ધાતુના વેલ્ડિંગમાં ઉપયોગી છે.
- 54View Solutionસાદામાં સાદો આલ્કાઇન..... દ્વારા દર્શાવાય છે ?
- 55View Solutionહાઇડ્રોકાર્બન ઉદ્દીપકીય રિડકશનમાં મોટેભાગે કયો ઉદ્દીપક વપરાય છે ?
- 56$100$ ઓક્ટેન આંક ............. ને આપવામાં આવે છે.View Solution
- 57$1$-ફિનાઈલ પ્રોપીનમાં $HCl$ ઉમેરવાથી શું મળે છે ?View Solution
- 58$1$-બ્રોમો-$3$-ક્લોરો સાયક્લો બ્યુટેન ઉપર ઈથરમાં $2$ તુલ્યતા ધરાવતા સોડિયમ સાથે પ્રક્રિયા કરતા શું આપે છે ?View Solution
- 59$1$-મિથાઈલ સાયક્લો હેકઝીન એ $B_2H_6$ સાથે પ્રક્રિયા કરે છે. નીપજને $H_2O_2$ અને $NaOH$ સાથે પ્રક્રિયા કરવામાં આવે છે. પ્રક્રિયા એ નીપજ શું હશે ?View Solution
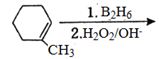
- 60$21$ ગ્રામ $C_3H_6$ સાથે બ્રોમિનની પ્રક્રીયા થવા માટે કેટલા......$g$ ગ્રામ બ્રોમિનની જરૂર પડે ? ($Br$ નો પરમાણુભાર = $80$)View Solution
- 61$2$-બ્રોમોબ્યુટેનમાંથી હાઈડ્રોજન બ્રોમાઈડના વિલોપનના પરીણામે .......નું નિર્માણ થાય છે ?View Solution
- 62$3$-ઓકટાયનનું સંશ્લેષણ એ બ્રોમો આલ્કેનમાં સોડિયમ એમાઈન અને આલ્કાઈનના મિશ્રણને ઉમેરવાથી થાય છે. આ બ્રોમો આલ્કેન અને આલ્કાઈન કયો હશે?View Solution
- 63$3$-ફિનાઈલ પ્રોપીન પર $HBr$ સાથે પ્રક્રિયા કરતા (મુખ્ય નીપજ) શું મળે છે ?View Solution
- 64$40°$ સે. એ $HBr$ નો એક અણુ $1,3$-બ્યુટાઈનના એક અણુ સાથે પ્રક્રિયા કરીને શું બનાવે છે ?View Solution
- 65$725\,^oC$ તાપમાને પ્રકાશની હાજરીમાં $n$ -બ્યુટેનની બ્રોમિન સાથેની પ્રક્રિયાથી ................... મળે છેView Solution
- 66$80\% \,D_2SO_4$ માં $D_2O$ ના વધુ પ્રમાણમાં ગરમ બેંઝિન કઇ નીપજ પરિણમે છેView Solution
- 67$(A)$ અને $(B)$ સમઘટકો છે નીપજ $(B)$ શું હશે :ડેવાર બેંઝિનView Solution
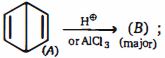
- 68$C_2H_5I$ ની ઇથરની હાજરીમાં $Na$ સાથેની પ્રક્રિયાથી બ્યુટેનની બનાવટમાં ........... ની અશુદ્ધિ સંકળાયેલી છે.View Solution
- 69$C_6H_{10}$ હાઈડ્રોકાર્બન ઉદ્દીપક હાઈડ્રોજીનેશનમાં $H_2$ નો માત્ર એક અણુનું શોષણ કરે છે. જેના પર ઓઝોનોલીસીસ કરતા, હાઈડ્રોકાર્બનની ઉપજ $CHO(CH_2)_4CHO$ નીપજ આવે છે. હાઈડ્રોકાર્બન કયો છે ?View Solution
- 70$Ca{C_2} + {H_2}O \to A\xrightarrow{{{H_2}S{O_4}/HgS{O_4}}}B$ પ્રક્રિયામાં $A$ અને $B$ શુ છે ?View Solution
- 71$CCl_4$ માં પ્રોપીન ની $N$ - બ્રોમો સકિસનેમાઈડ સાથે પ્રક્રિયા કરતા શું મળે છે ?View Solution
- 72$CH_2 = CH_2$ ના ઓઝોનોલિસિસ દરમિયાન જો $LiAlH_4$ વડે રિડક્શન કરવામાં આવે તો મળતી નીપજ .............. થશે.View Solution
- 73$CH_2 = CH_2$ ના ઓઝોનોલિસિસ દરમિયાન જો $Zn\, dust$ ની ગેરહાજરીમાં જળવિભાજન કરવામાં આવે તો મળતી નીપજ ............... થશે.View Solution
- 74$CH_2 = CH-(CH_2)_8COOH + HBr$ $\xrightarrow{{Peroxide}}$ ......... પ્રક્રિયાની મુખ્ય નીપજ ............... થશે.View Solution
- 75$(CH_3)_2 C = CH-CH_3$ સંયોજનની $KMnO_4$ ની હાજરીમાં $NaIO_4$ સાથેની પ્રક્રિયાથી ......મળે છે.View Solution
- 76$(CH_3)_2CH - C = C -CH_3$ નુ ઓઝોનોલિસિસ $(O_3, H_2O)$........... આપે છે.View Solution
- 77$C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\xrightarrow{C{{H}_{2}}{{N}_{2}}/\Delta } $ નીપજView Solution
ઉપરોક્ત પ્રકિયા માં કઈ નીપજ મળતી નથી
- 78$C{H_3}CH\, = \,\,CHC{H_3}\,\xrightarrow{{{O_3}\,}}\,\,A\,\,\mathop {\xrightarrow{{{H_2}O}}}\limits_{Zn} \,\,B.$ પ્રક્રિયા શ્રેણીમાં આલ્કીન પદાર્થ $B$ બનાવે છે. પદાર્થ $B$ કયો છે?View Solution
- 79$CH_3 - C \equiv C - CH_2CH_3$ $\xrightarrow[{\left( {ii} \right)\,Hydrolysis}]{{\left( i \right)\,{O_3}}}$ પ્રક્રિયાની નીપજો ..... થશે.View Solution
- 80$CH_3-C \equiv C - CH_3$ $\xrightarrow{{NaN{H_2}}}$ $X$ તો $X$ =.........View Solution
- 81$C{H_3} - \mathop {CH}\limits^{\mathop |\limits^{C{H_3}} } \,\, - \,\,C{H_2} - C{H_3}\,\,\xrightarrow{{C{l_2}/hv}}\,\,N$( સમઘટકોની સંખ્યા) $ \xrightarrow{{Fractional\,\,\,{\text{distillation}}}}\,\,(F)\,,\,\,(N)$ અને $\,(F)\,$ એ .............View Solution
- 82$CH \equiv CH\,\,\mathop {\xrightarrow{{H{g^{ + 2}}}}}\limits_{{H_2}S{O_4}} \,\,B\,\xrightarrow{{C{H_3}MgX/{H_2}O}}\,\,C\,\,\xrightarrow{{[O]}}\,D$ અંતિમ નીપજ $D$ શું હશે ?View Solution
- 83$C.N.G$ એ........View Solution
- 84$FeBr_3$ ની હાજરીમાં ઇથાઇલ બેન્ઝિનની બ્રોમિન સાથેની પ્રક્રિયાથી ... મળે છે.View Solution
- 85${H_2}\,C = \,\,CH{(C{H_2})_6}\, - \,\,C{H_3}\,\mathop {\xrightarrow{{peroxide}}}\limits_{HBr} \,\,$ પ્રક્રીયાની નીપજ શું હશે ?View Solution
- 86$HBr$ સાથેની યોગશીલ પ્રક્રિયામાં નીચેના સંયોજનોની પ્રતિક્રિયાત્મકતા જણાવોView Solution
- 87View Solution(image) ના ઓઝોનોલિસિસથી .......... મળે છે.
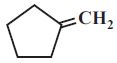
- 88View Solution(image) નુ ઓઝોનોલિસિસ .......... આપે છે
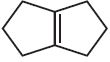
- 89$KBr$ ના સંતૃપ્ત દ્રાવણમાં ઇથિનની $Cl_2$ સાથેની પ્રક્રિયાથી............... મળે છે.View Solution
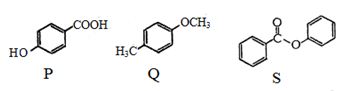
- 90$P, Q$ અને $S$ સંયોજનમાં અલગ અલગ જગ્યાએ $HNO_3/H_2SO_4$ ના મિશ્રણનો ઉપયોગ કરીને નાઇટ્રેશન કરતા દરેક કિસ્સામાં મુખ્ય નિપજ શું મળે છે ?View Solution
- 91$p$-નાઇટ્રોફીનોકસાઇડ આયનના સસ્પંદન બંધારણના સૌથી અસમાન નિરુપણ છે ?View Solution
- 92$R\,-\,C{{H}_{2}}\,-\,C\,\cdot \,C{{l}_{2}}\,-\,R\,\xrightarrow{activator}\text{R}\,\text{-}\,\text{C}\,\equiv \,\text{C}\,\text{-}\,\text{R}$ પ્રક્રિયક ...... છે.View Solution
- 93$R-CH_2CCl_2-R$ $\xrightarrow{{\operatorname{Re} agent}}$ $R-C \equiv C - R$ પ્રક્રિયામાં વપરાતો પ્રક્રિયક ............. છે.View Solution
- 94$X $ ઉપર ઓઝોનોલીસીસ કરતા અને પછી રીડકશન કરતા બે મોલ સમાન આલ્ડીહાઈડ આપે છે તો $ 'X'$ શું હશે ?View Solution
- 95View Solutionઅમુક આલ્કેનને ક્લોરિન સાથે મિશ્ર કરી તેના પર પારજાંબલી વિકિરણો વિકરિત કરવામાં આવે છે ત્યારે માત્ર એક જ મોનોક્લોરો આલ્કેન બને છે.આ આલ્કેન શક્યતા પ્રમાણે.....હોઇ શકે.
- 96આપેલ પ્રક્રિયા કયા નામે ઓળખાય છે ?$C{H_3}CH = C{H_2}\mathop {\xrightarrow{{(CO + {H_2})}}}\limits_{{H^{^ + }}} C{H_3} - \mathop {\mathop {CH - C{H_3}}\limits_{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,} \,\,\,\,\,}\limits_{COOH\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,} \,\,$View Solution
- 97આપેલ પ્રક્રિયામાં $‘X’$ શું છે. ? $HC \equiv CH + 2AgN{O_3}\xrightarrow{{N{H_4}OH}}X + 2N{H_4}N{O_3} + 2{H_2}O$View Solution
- 98ઇથાઇલ બ્રોમાઇડ તથા $n-$ પ્રોપાઇલ બ્રોમાઇડની ઇથરમાંના સોડિયમ સાથેની પ્રક્રિયાથી ................. મળે છે.View Solution
- 99View Solutionઇથિલિનની ..... સાથેની પ્રક્રિયાથી ઇથિલિન ક્લોરોહાઇડ્રીન બને છે.
- 100View Solutionઉપરની પ્રક્રિયાઓની સક્રિયકરણ ઉર્જાઓ વચ્ચેનો સંબંધ કયો છે